✭ FAMILIES OF ORGANIC COMPOUNDS
✩1- Introduction to Alkanes
Introduction to Organic Chemistry/ Alkanes
Part 1 - Carbon - Recap
Why can carbon form so many compounds?
What we know about carbon
1s22s22p2
Four valence electrons
Non-metal
How many bonds can it form?
2, 3 or 4
What type of bonds can it form?
Covalent
Single or multiple
What atoms can it bond to?
Many
Commonly other carbon, hydrogen, nitrogen, oxygen, sulfur, halogens
Part 2 - Hydrocarbons - Main Focus on Acyclic Alkanes and Alkenes
◻ Background Info:
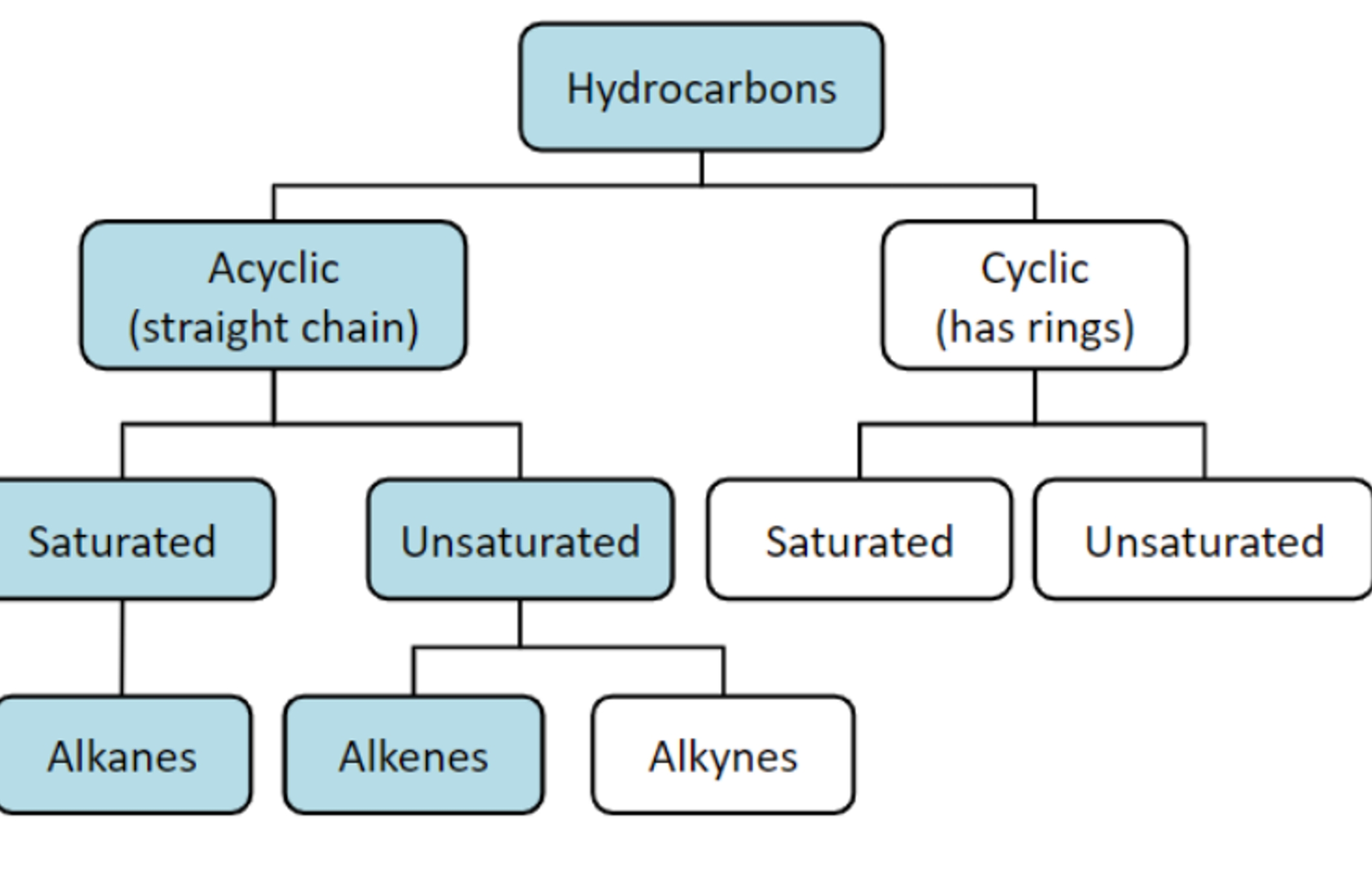
Are the simplest organic molecules as they only contain hydrogen and carbon yet they vary in complexity and so are simplified by grouping.
◻NAMING HYDROCARBONS
Hydrocarbon use prefixes and suffices
Prefix → Number of carbons (Prop for 3 etc)
Suffix → Type of Hydrocarbon (“-ane” for alkanes, “-ene’ for alkenes)

![]()
◻ AKLANES

The simplest alkane contains one carbon


Alkanes are the simplest [hydrocarbon] because they only have single carbon-carbon (c-c) bonds. Alkanes contain the maximum possible number of atoms and are said to be saturated.
◽ Alkanes are saturated
Breaking down the sentence:
“maximum possible number of atoms” → This refers to the fact that each carbon atom in an alkane forms four single covalent bonds (either with hydrogen atoms or other carbon atoms). This configuration maximizes the number of hydrogen atoms attached to the carbon skeleton.
“saturated” → In chemistry, when a molecule contains the maximum possible number of hydrogen atoms (with no double or triple bonds between carbon atoms), it is said to be "saturated." This term means that the molecule cannot accommodate any more hydrogen atoms without breaking the existing bonds.
So, putting it all together:
◽ Alkanes are saturated because
Alkanes are hydrocarbons with only 4 single bonds (covalent)…
Carbons in the alkanes are bonded to the maximum amount of atoms.
Because of this, alkanes are said to be "saturated" with hydrogen.
◽Naming Alkanes
Prefix → number of carbons
Suffix → type of hydrocarbon ( -ane for alkanes)
◽Building the Alkane Series
Be able to write
[x] Methane
[x] Ethane
[x] Propane
◽General Formula For Alkanes
CnH2n+2
◽Homologous Series
(Alkanes as a homologous series) where each successive member differs by a CH2 unit (a methylene group)
Info: Alkanes fall into a family that reacts similarly when interacting with other chemicals because of similar chemical properties
Homologous series: A group of organic compounds with similar general formula, chemical properties, and a graduation in physical properties where each successive series differs by a specific repeating unit.
Examples:
Methane (CH₄): The simplest alkane, with one carbon atom.
Ethane (C₂H₆): The next alkane, with two carbon atoms. It can be seen as methane plus one CH₂ unit.
Propane (C₃H₈): Another alkane, with three carbon atoms. It can be seen as ethane plus one CH₂ unit.
Butane (C₄H₁₀): Following the pattern, butane has four carbon atoms, which can be seen as propane plus one CH₂ unit.
✩2- Different Representations of Organic Compounds and Introduction
Different Representation of Organic Compounds and Intro to Structural Isomers
Learning outcomes
[ ] To demonstrate how to represent alkanes using structural formulas, and semi-structural formulas.
[ ] Identify the difference between a straight chain and a branched chain alkane
[ ] Define what a structural isomer is.
◽REPRESENTING HYDROCARBONS

Drawing Semistructural Formuals for:
Ethane: CH3-CH3
Pentane: CH3-CH2-CH2-CH2-CH3
Heptane: CH3-CH2-CH2-CH2-CH2-CH2-CH3
◽ STRAIGHT CHAIN ALKANES VS BRANCHES CHAIN ALKANES
Straight Chain: All the carbons follow each other continuously
Branched Chain Alkanes: Not all the carbons follow each other continuously. (i.e there’s a parent chai and branched chain(s)


◽ STRUCTURAL ISOMERS
Structural isomers are molecules with the same molecular formula but different arrangements of their atoms
They have
similar chemical properties
different physical properties
Straight-chain isomers tend to have higher boiling temperatures than branched chain isomers.
✩3- Isomers of Alkanes
Branching and isomers
learning outcomes:
[x] Demonstrate the ability to name branched non-cyclic hydrocarbon compounds (up to C8) and structural isomers (up to C5) according to IUPAC systematic nomenclature.
[x] Draw Structural Isomers of Alkanes
C5 and C8 meaning
"C8" and "C5": These notations refer to the number of carbon atoms in the hydrocarbon molecules. "C8" means a hydrocarbon with up to 8 carbon atoms, and "C5" means a hydrocarbon with up to 5 carbon atoms.
◻ NAMING STRUCTURAL ISOMERS OF ALKANES

Steps:
Identify the longest carbon chain + side chain
Number carbons from the end that give the side chain the lowest number possible

#Note: Naming side chains
Hydrocarbon side chains are called ‘alkyl’
Prefix is used to indicate the number of times a particular side chain appears
Side chains of the SAME type are grouped and the position of each is shown (2,3-dimethylbutane)
Side chains of DIFFERENT types are listed in alphabetical order (3-ethyl-2-methylpentane)

✩4-Alkanes
alkanes
learning outcomes:
[ ] Name and draw Structural and Semi-Structural formula for Alkenes up to 8 carbon atoms
[ ] Write the General formula for an alkene
[ ] Identify that alkenes are unsaturated and be able to explain this term
[ ] Name and draw branched chain alkenes
[ ] Name and draw isomers for alkenes up to 5 carbon atoms
◻ ALKENES

They are hydrocarbons that contain one or more carbon-to-carbon double bonds.
Since each carbon atom is no longer bonded to the maximum possible number of atoms - alkenes are unsaturated hydrocarbons.
General Formula for alkenes: CNH2N
Naming alkenes
suffix “ene”
show the position of double bond
name carbons starting at end closet to the double bond
number of the first C that has double bonds is inserted between the prefix and suffix.
✩5-Functional Groups
functional groups
learning outcomes
[ ] Be able to identify different functional groups, including, haloalkane, hydroxyl, and carboxyl groups and be able to name and draw molecules containing these.
FUNCTIONAL GROUPS
A functional group is an atom or group of atoms that has similar chemical properties when it occurs in different compounds. It defines the characteristic physical and chemical properties of families in an organic compound
#Note 1: slide 57
Alkanes are not considered functional groups, instead a compound that lacks functional groups. However, the C-C double bond in carbons is considered a functional group.??
Alkanes are not usually considered as functional groups; instead, an alkane is a compound that lacks functional groups. The C-C double bond in carbons, however, is considered a functional group.
HALOGENS
Has a carbon single bonded to a halogen (X=F,Cl,Br,I)
Use the prefix halo- (i.e. fluoro-, chloro-,bromo-, or iodo-)
(important to remember that the halogen is considered of equal rank with an alkyl group in the number of parent chains so the
rule of side groups having to be numbered the lowest is applied
if they are the same then which is alphabetically first is first)
HYDROXYL GROUP

Hydroxyl groups:
Molecules that contain a hydroxyl functional group are termed alcohols or alkanols
contains OH
uses the suffix “-ol” in naming (shows the position of the -OH using number before the suffix
hydroxyl groups take priority over halogens and alkyl groups
CARBOXYL GROUP

Molecules that contain a carboxyl group are termed carboxylic acids
contain -C=O(OH)
uses the suffix “-oic acid” (always on the final carbon in the chain)
carboxyl groups take priority over hydroxyl groups, halogens, and alkyl groups.
FUNCTIONAL GROUPS SUMMARY
A functional group is an atom or group of atoms with similar chemical properties in different compounds, defining the physical and chemical properties of families in an organic compound.
✩6- Properties of organic compounds
properties of organic compounds
learning outcomes
[ ] To understand the physical properties of hydrocarbons.
[ ] using their understanding of covalent bonding, polarity, and intermolecular forces.
[ ] Explain what happens to mp and bp of branched-chain isomers vs straight chain isomers
[ ] Identify the strongest intermolecular forces present in functional groups and use that to determine the link between melting and boiling points.
◻HOMOLOGOUS SERIES
Members of a homologous series have
similar structure
pattern to physical properties and similar chemical properties
same general formula
Q: How does an increase in CH2 group change chemical and physical properties?
H: Intermolecular forces (hydrogen, dipole-dipole, dispersion forces) and chemical reactivity across single &double, triple bonds.
With increasing molecular mass both melting and boiling points increase while solubility in water tends to decrease
PHYSICAL PROPERTIES OF HYDROCARBON
Property | Prediction and Justification |
|---|---|
Electrical conductivity | Generally non-conductive |
No free-moving charged particles | |
Behavior in an electric field | Not affected |
Non-polar | |
Solubility in polar solvents | Insoluble |
Hydrophobic | |
Malleability | The intermolecular forces (dispersion forces) between molecules are very weak, so a hydrocarbon solid can be bent out of shape relatively easily |
Melting temperature | Relative low melting and boiling points |
Intermolecular forces disrupted |
PROPERTIES OF ALKANES: BOILING AND MELTING

As the molecular mass increases, the strength of dispersion forces increases because there are more opportunities for instantaneous dipoles to form with increasing molecular size
how does branching affect melting temperature

With a greater surface area for contact between molecules, the stronger the dispersion forces become. In general, the more branches that a molecule has, the smaller its area of contact with other molecules and therefore the weaker the dispersion force.

FACTOR THAT AFFECTS MELTING AND BOILING POINT

If comparing two straighter chained molecules of different sizes, the larger molecule (larger molecular mass) will have the higher melting/boiling point.
If comparing a straight-chain molecule with a branched molecule of similar size the straight-chain molecule will have a higher melting/boiling point.