Fire and Explosives
Fire and Explosive Investigation
Arson investigations often present complex and difficult circumstances to investigate:
The perpetrator has thoroughly planned the act.
The perpetrator is not present during the act.
The destruction is so extensive.
Oxidation
Chemically, fire is a type of oxidation, which is the combination of oxygen with other substances to produce new substances.
Not all oxidation reactions proceed in a manner that one associates with a fire; e.g., rusting.
An oxidation reaction is associated with the concept of energy.
Energy is the ability of a system to do work.
Steam can turn a turbine to generate electrical energy.
energy takes many forms; e.g., heat and light.
All oxidation reactions are examples in which more energy is liberated than what is required to initiate the reaction.
These are known as exothermic reactions.
Combustion
To start a fire, the ignition point (minimum temperature needed to ignite fuel spontaneously) must be reached.
The heat and light released when a substance burns is known as heat of combustion.
Once combustion starts, energy in the form of heat and light (flame) is liberated. A portion of this energy is used to sustain the fire.
Fire is a chain reaction.
To initiate and sustain a fire, the following are required:
A fuel must be present.
Oxygen must be available in sufficient quantity to combine with the fuel.
Heat must be applied to initiate the combustion, and sufficient heat must be generated to sustain the reaction.
Physical state of the fuel
A fuel achieves a reaction rate with oxygen sufficient to produce a flame only when it is in the gaseous state. Thus, rusting is not accompanied by a flame.
A liquid burns when the temperature is high enough to vaporize the fuel.
The flash point is the lowest temperature at which a liquid produces enough vapor to burn.
i.e. gasoline has a flash point of -40°C
A solid such as wood burns only when exposed to heat hot enough to decompose it into gaseous products (pyrolysis).
Glowing combustion, or smoldering, is burning located at the fuel-air interface, such as a cigarette, the embers of a wood fire, or a charcoal fire.
Heat Transfer
Conduction
the movement of heat through a solid object.
Poor conductors are called insulators.
During a fire, heat may transported through metals, such as nails, bolts, and fasteners to a location far from the initial heat source, creating a new fire location.
Radiation
is the transfer of heat energy by electromagnetic radiation.
A surface exposed to the heat of a fire may burst into flames when the surface reaches the ignition temperature.
paper at 451°F
Convection
is the transfer of heat energy by the movement of molecules within a liquid or gas.
Like a convection oven
In a structural fire, hot gases move to the upper portion of the structure causing surfaces to pyrolyze and burst into a fire
The Fire Scence
The arson investigator needs to begin examining a fire scene for signs of arson as soon as the fire has been extinguished.
Most arsons are started with petroleum-based accelerants.
gasoline, lighter fluid, kerosene, turpentine, butane, and others
The search of the fire scene must focus on finding the fire's origin, which may be most productive in any search for an accelerant or ignition device.
Indicators of arson
Evidence of separate and unconnected fires
The use of "streamers" to spread the fire from one area to another
An irregularly shaped pattern on the floor resulting from the pouring of accelerant onto the surface
Normally, a fire has a tendency to move in an upward direction. Thus the probable origin will most likely be the lowest point showing the most intense characteristics of burning.
Evidence of severe burning found on the floor (as opposed to the ceiling) of a structure is indicative of a flammable liquid
Discovery of an ignition device:
The most common igniter is a match.
Arsonists can construct many other types of devices to start a fire, including burning cigarettes, firearms, ammunition, a mechanical match-striker, electrical sparking devices, and a “Molotov cocktail.”
Flashover
occurs when all the combustible fuels simultaneously ignite to engulf the entire structure.
A fire that starts in one area of a structure could, through flashover, create the illusion of more unrelated fires, a sign mistaken for arson.
Irregular patterns are common in post-flashover conditions.
If the presence of ignitable liquids is suspected to have caused a fire pattern, supporting evidence from the laboratory for the presence of accelerant residues must confirm its existence.
Many factors can contribute to the deviation of a fire from normal behavior.
Using burn patterns such as depth of char, a V-shaped pattern, or low intense burn area, as indicators of a fire's origin can prove to be misleading when a flashover has occurred.
Searching for Accelerants
Combustible liquids are rarely entirely consumed in a fire.
The search for traces of flammable liquid residues may be aided by the use of a sensitive portable vapor detector or “sniffer.”
Collection of fire scene evidence
At the suspect point of origin of a fire, collect ash, soot, and porous materials which may contain excess accelerant.
Store them in airtight containers such as new paint cans or wide-mouth glass jars, leaving an airspace to remove samples.
Never use plastic containers to store fire scene evidence.
The collection of all materials suspected of containing volatile liquids must be accompanied by a thorough sampling of similar but uncontaminated control specimens from another areas of the fire scene, called a substrate control.
Laboratory recovery of flammable residues
The easiest way to recover accelerant residues from fire-scene debris is to heat the airtight container in which the sample is sent to the laboratory.
When the container is heated, any volatile residue in the debris is driven off and trapped in the container's enclosed airspace.
The vapor or headspace is then removed with a syringe.
When the vapor is injected into the gas chromatograph, it is separated into its components, and each peak is recorded on the chromatogram.
In the vapor concentration technique, a charcoal strip is placed in the airtight debris container when it is heated.
The charcoal strip absorbs much of the vapors during heating.
The strip is washed with a solvent which will recover the accelerant vapors.
The solvent is then injected into the gas chromatograph for analysis.
Gas Chromatography
In the laboratory, the gas chromatograph is the most sensitive and reliable instrument for detecting and characterizing flammable residues.
The vast majority of arsons are initiated by petroleum distillates such as gasoline and kerosene.
The gas chromatograph separates the hydrocarbon components and produces a chromatographic pattern characteristic of a particular petroleum product
By comparing select gas chromatographic peaks recovered from fire-scene debris to known flammable liquids, a forensic analyst may be able to identify the accelerant used to initiate the fire.
The chromatographic pattern of the unknown is compared to patterns produced by known petroleum products.
Accelerant Identification
Typically a forensic analyst compares the pattern generated by the sample to chromatograms from accelerant standards obtained under the same conditions.
The pattern of gasoline, as with many other accelerants, can easily be placed in a searchable library.
An invaluable reference known as the Ignitable Liquids Reference Hydrocarbon Collection (ILRC)
Complex chromatographic patterns can be simplified by gas chromatography/mass spectrometry.
Explosions
Explosives are substances that undergo a rapid oxidation reaction, producing large quantities of gases.
It is this sudden buildup of gas pressure that constitutes the nature of an explosion.
Explosives can be classified as high or low explosives
Low Explosives
The most widely used low explosives are black powder and smokeless powder.
Black powder is a mixture of potassium or sodium nitrate, charcoal, and sulfur.
Smokeless powder consists of nitrated cotton (nitrocellulose) or nitroglycerin and nitrocellulose
Low explosives are often confined to a container like a pipe.
The speed of decomposition in a low explosive is called deflagration, causing the walls of the container to fragment and fly outward in all directions.
High Explosives
Primary explosives
ultra-sensitive to heat, shock, or friction
provide the major ingredients found in blasting caps or primers used to detonate other explosives.
Secondary explosives
relatively insensitive to heat, shock, or friction and will normally burn rather than detonate if ignited in small quantities in the open air.
This group comprises the majority of commercial and military blasting, such as dynamite, TNT, PETN, and RDX.
MUST be detonated by a primary explosive.
The speed of decomposition of high explosives is known as detonation. It’s extremely rapid, producing a supersonic shock wave creating a blast effect with an outward rush of gases at speeds as high as 7,000 miles per hour.
In recent years, nitroglycerin-based dynamite has all but disappeared from the industrial explosive market and has been replaced by ammonium nitrate-based explosives.
Military and Peroxide Explosives
Triacetone triperoxide (TATP) is a homemade explosive that has been used by terrorist organizations.
can be made by combining acetone and peroxide in the presence of an acid.
Its existence has led to the banning of most liquids on commercial aircrafts.
In many countries outside the United States, the accessibility of military high explosives to terrorist organizations makes them very common constituents of homemade bombs.
RDX is the most popular and powerful of the military explosives, often encountered in the form of pliable plastic known as C-4.
Collection and Analysis
The entire bomb site must be systematically searched, with great care given to recovering any trace of a detonating mechanism or any other item foreign to the explosion site.
Objects located at or near the origin of the explosion must be collected for laboratory examination.
Often a crater is located at the origin and loose soil and other debris must be preserved from its interior for laboratory analysis.
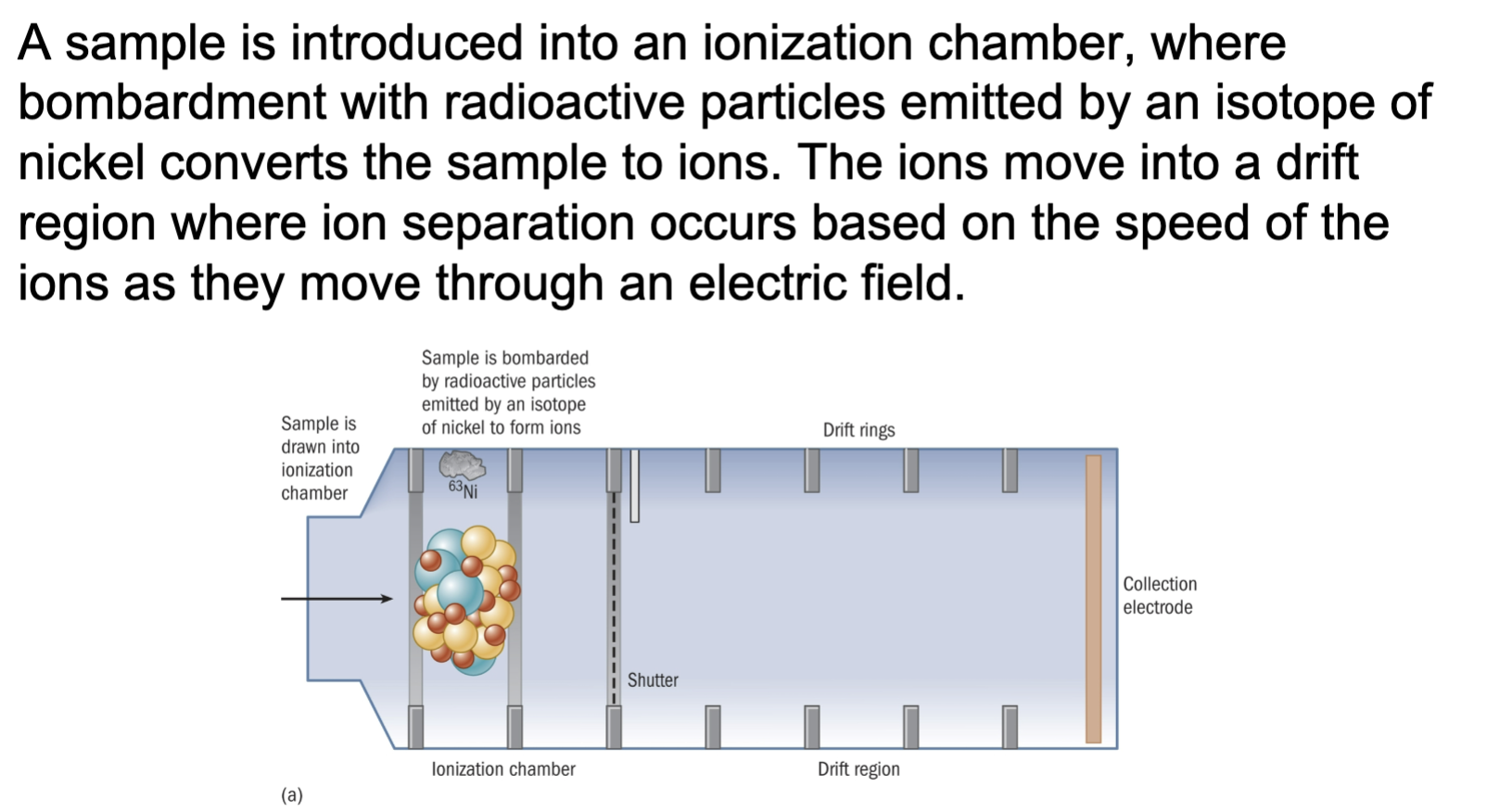
One approach for screening objects for the presence of explosive residues is the ion mobility spectrometer.
All materials collected for the examination by the laboratory must be placed in sealed air-tight containers and labeled with all pertinent information.
Debris and articles collected from different areas are to be packaged in separate air-tight containers.
Some explosives can diffuse through plastic and contaminate nearby containers.
At the Lab
Typically, in the laboratory, debris collected at explosion scenes will be examined microscopically for unconsumed explosive particles.
Recovered debris may also be thoroughly rinsed with organic solvents and analyzed by testing procedures that include color spot tests, thin-layer chromatography, and gas chromatography/mass spectrometry.
Confirmatory identification tests may be performed on unexploded materials by infrared spectrophotometry.