Amino Acid Metabolism in Biochemistry (PPT)
Chapter 17: Amino Acid Metabolism
17.1 Nitrogen Fixation and Assimilation
Nitrogen Fixation: Conversion of atmospheric nitrogen (N₂) to ammonia (NH₃) or ammonium (NH₄⁺).
Occurs through bacteria (biological), the Haber process (industrial), or lightning (atmospeheric).
Nitrogen Assimilation: Incorporation of NH₄⁺ into amino acids.
Done primarily by plants and bacteria using the enzyme glutamine synthetase.
Biological and Industrial Fixation
Biological Fixation:
Involves the nitrogenase enzyme to break the triple bond of N₂.
An ATP-dependent process consisting of six steps.
Industrial Fixation (Haber Process):
Involves reacting N₂ from the air and H₂ from methane at 500 °C and 350 kPa with an Fe catalyst.
Produces ammonia gas used in fertilizers and other chemicals.
Atmospheric Fixation
Lightning can oxidize N₂ to nitrogen oxides (i.e., NO) which can dissolve in rain, contributing to nitrogen sources in soil.
The Nitrogen Cycle
Maintains the nitrogen balance within ecosystems through various processes including fixation, assimilation, nitrification, and denitrification.
Bacteria such as Sinorhizobium and Azotobacter play significant roles in nitrogen fixation.
Nitrogen Assimilation
Glutamate and glutamine are key amino acids in providing nitrogen.
Glutamate transfers alpha amino group to alpha-keto acids to make any amino acids using specific amino transferases for each amino acid (source for biosynthesis reactions through amino transferase enzymes)
Glutamine is the primary source of amino groups to make amino acids, nucleotide bases, carbamoyl phosphate, etc.
Sources of nitrogen include ammonium (NH₄⁺) and ammonia (NH₃).
Key enzymes involved include:
Glutamine synthetase (found in all organisms)
Glutamate synthase (only in bacteria, plants, and some insects)
Glutamate dehydrogenase (Plants animals must deaminate; active in high concentrations of NH4+)
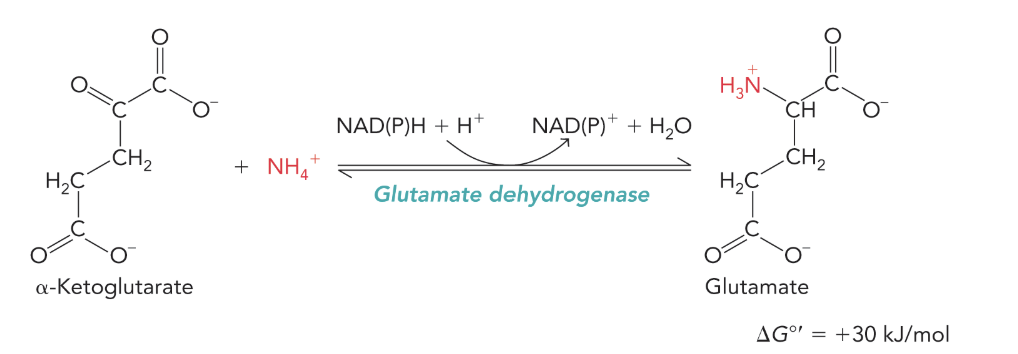
More likely to go in the reverse reaction. Need very high concentrations of NH4+
Glutamine Synthetase and Glutamate Synthase
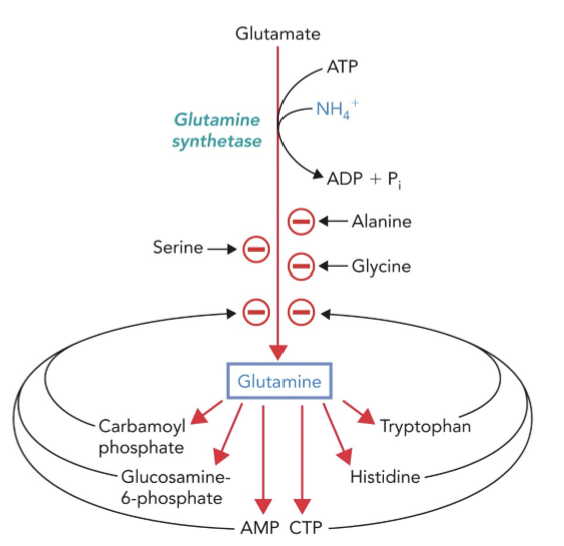
Glutamine synthetase: Converts glutamate to glutamine using 2 NH₄⁺ and ATP.
Critical for nitrogen transport from peripheral tissues to the liver where urea is formed.
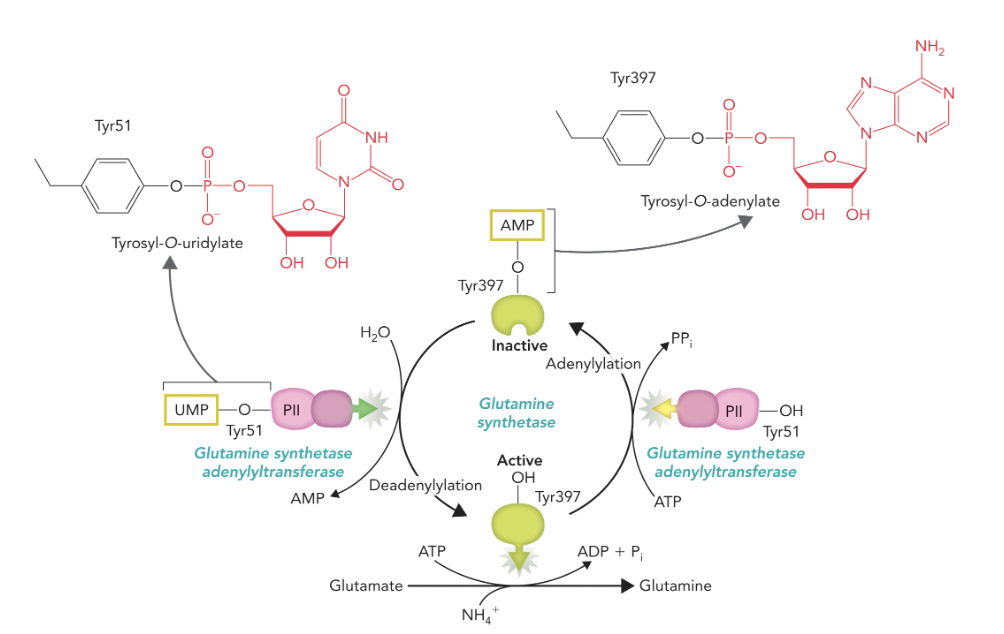
Regulated by covalent modifications (adenylations) and feedback inhibition (buildup of glutamine products like carbamoyl), and allosteric inhibitors; be able to justify how they regulate the glutamine synthetase
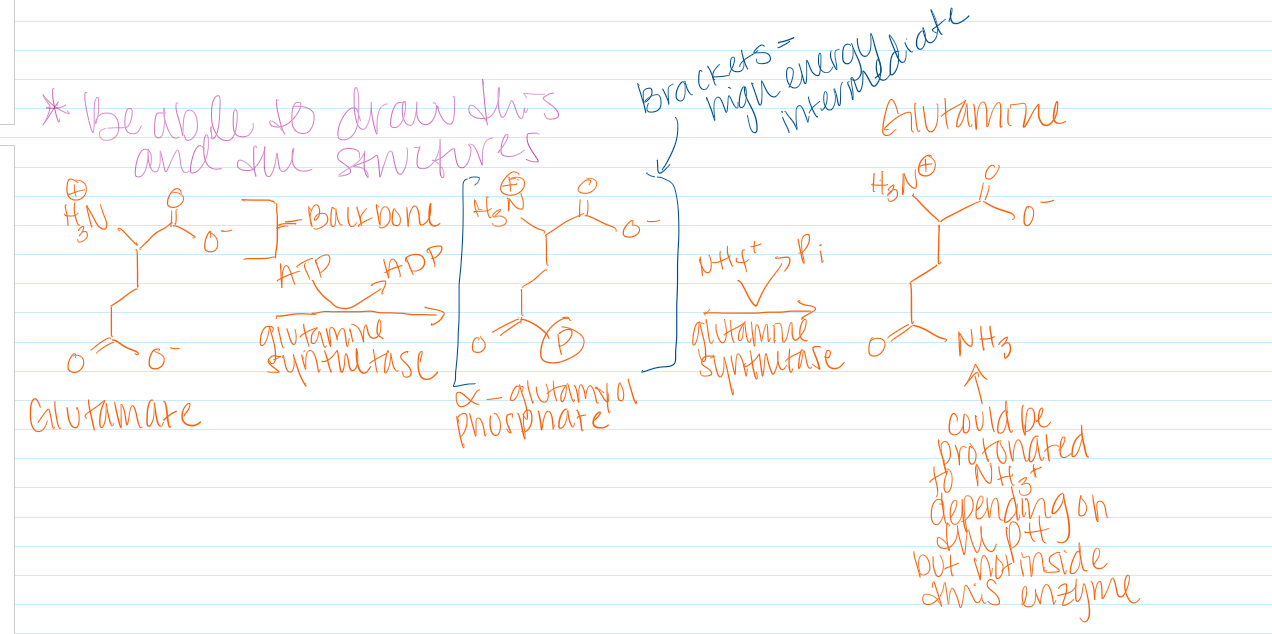
The -delta G from the PPi drives the reaction forward
Converts glutamine and alpha-ketoglutarate to glutamate by reducing NADPH or NADH
These can be reversible depending on concentrations and certain conditions
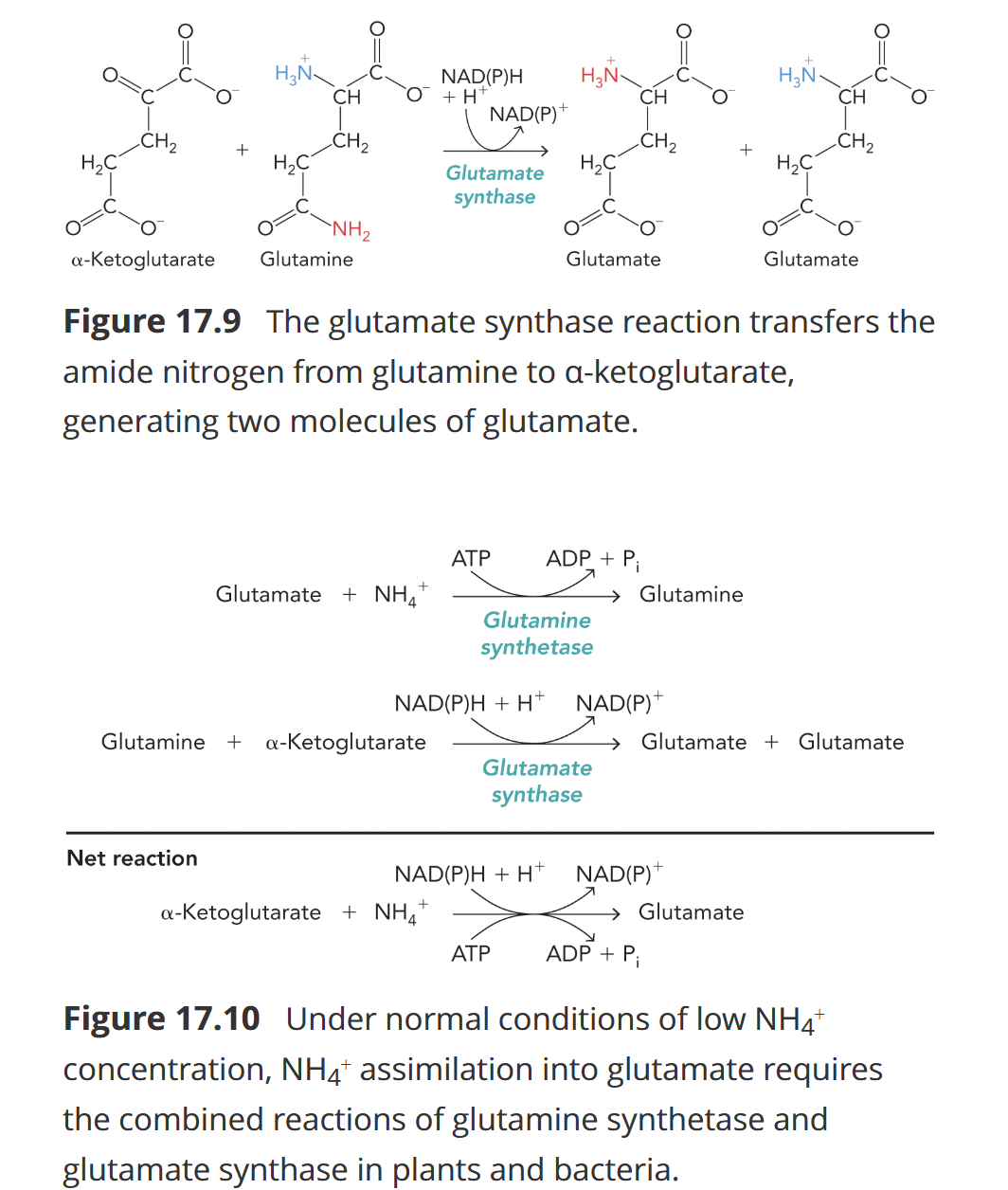
Technically makes 2 glutamate
Aminotransferases Mechanism
Aminotransferases transfer the α-amino group from an amino acid to an α-keto acid.
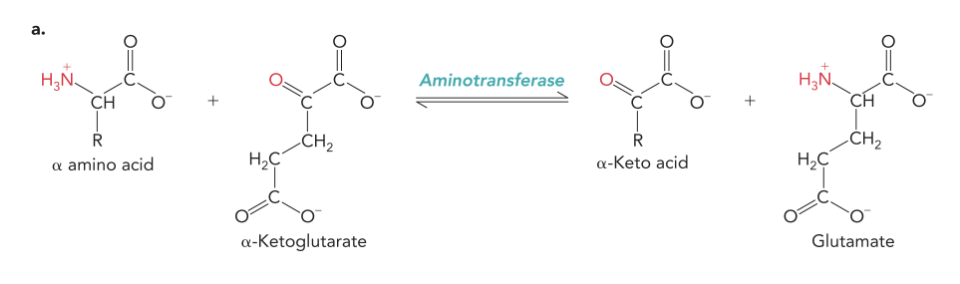
alpha ketoglutarate is the acceptor molecule that is accepting a NH4+
Most amino transferase reactions are reversible depending on the concentrations of the substrates (delta G ~ 0)
Pyridoxal phosphate (PLP) (coenzyme)is required for enzyme activity.
Stage 1 of mechanism: alpha amino group of an amino acid transferred to the amino transferase enzyme (ending with an alpha ketoacid). PLP picks up the amino acids and transfers the double bond from its ring to the amino acid so it will break off to form the alpha ketoacid
Stage 2: alpha amino group is transferred to a new amino acid
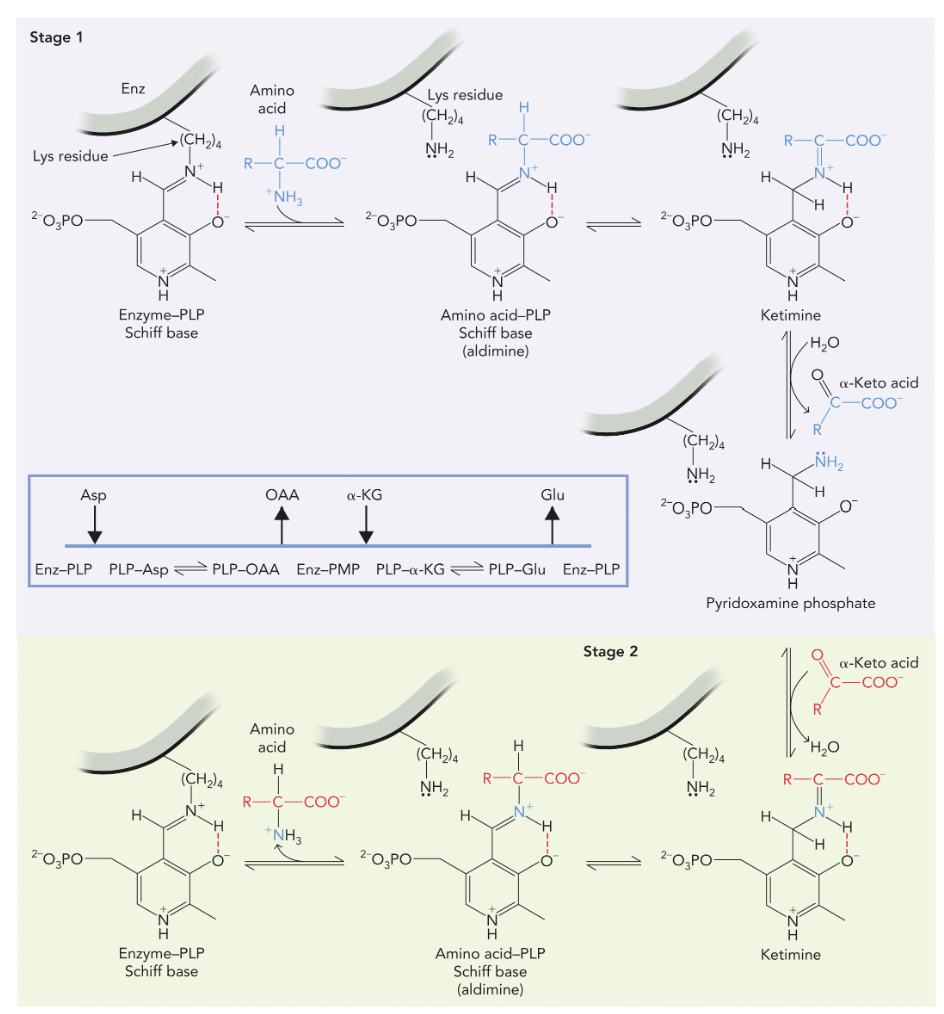
can interconvert any pools of amino acids
Aspartate and alanine aminotransferases indicator of liver health, high concertation = liver damage
17.2 Amino Acid Degradation
Degradation of proteins results in free amino acids and is a constant process for cellular function.
Same for nucleic acids (RNA is a big one)
To balance nitrogen you discard what you consume unless you are a child or pregnant women
nitrogen imbalance = disease or malnourished
Ammonium group transported from peripheral tissues to liver to start urea cycle for excretion
Carbon skeleton is a general energy conversion metabolite
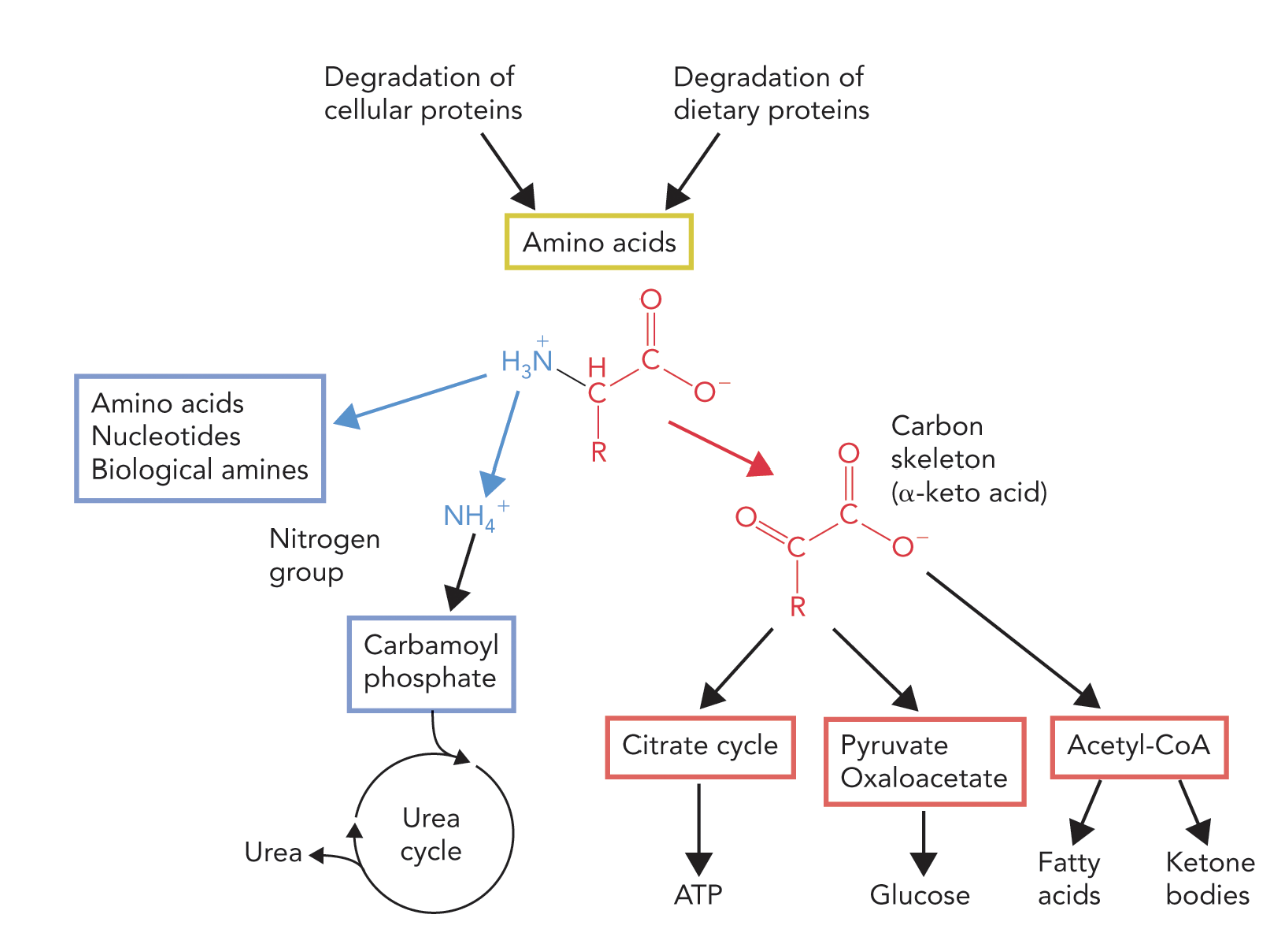
Dietary protein Digestion and Enzymes activation:
Gastrin production triggered when consumption of food
stimulates the secretion of zymogenes and HCl
Stomach and small intestine: enzymes (pepsin, proteases) start digestion; high acidity (changes the pronation state and denatures the proteins)
Sectrin stimulates the secretion of chime
Enzymes ends with -gen then it is a zymogene and must be cleaved to activate
pH triggers the conversion
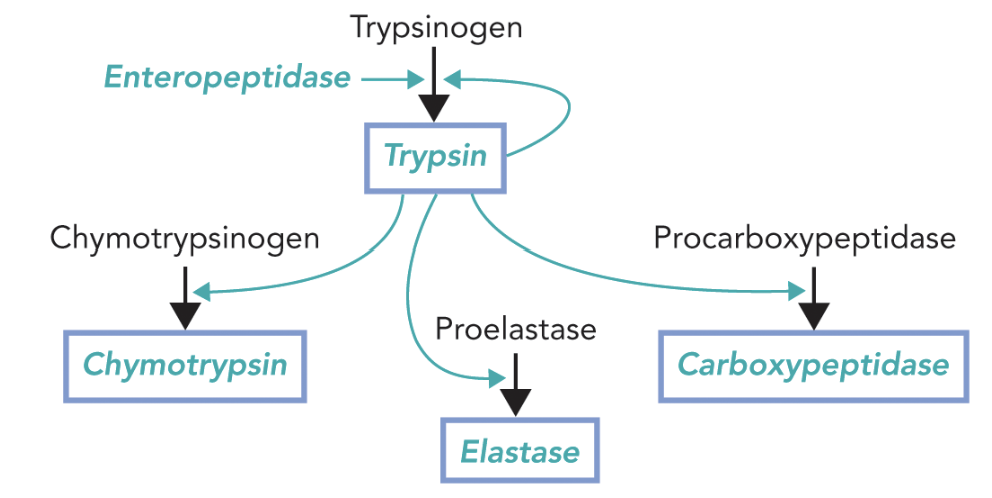
Different enzymes = different active sites that have different functions; not insanely specific but cleave certain groups of amino acids
Two pathways for cellular proteins degradation:
ATP-independent process (e.g., in lysosomes)
nonspecific lysosomal vesicles
Acidic proteases in the vesicles
ATP-dependent because of proton concentration but can eventually become ATP-independent
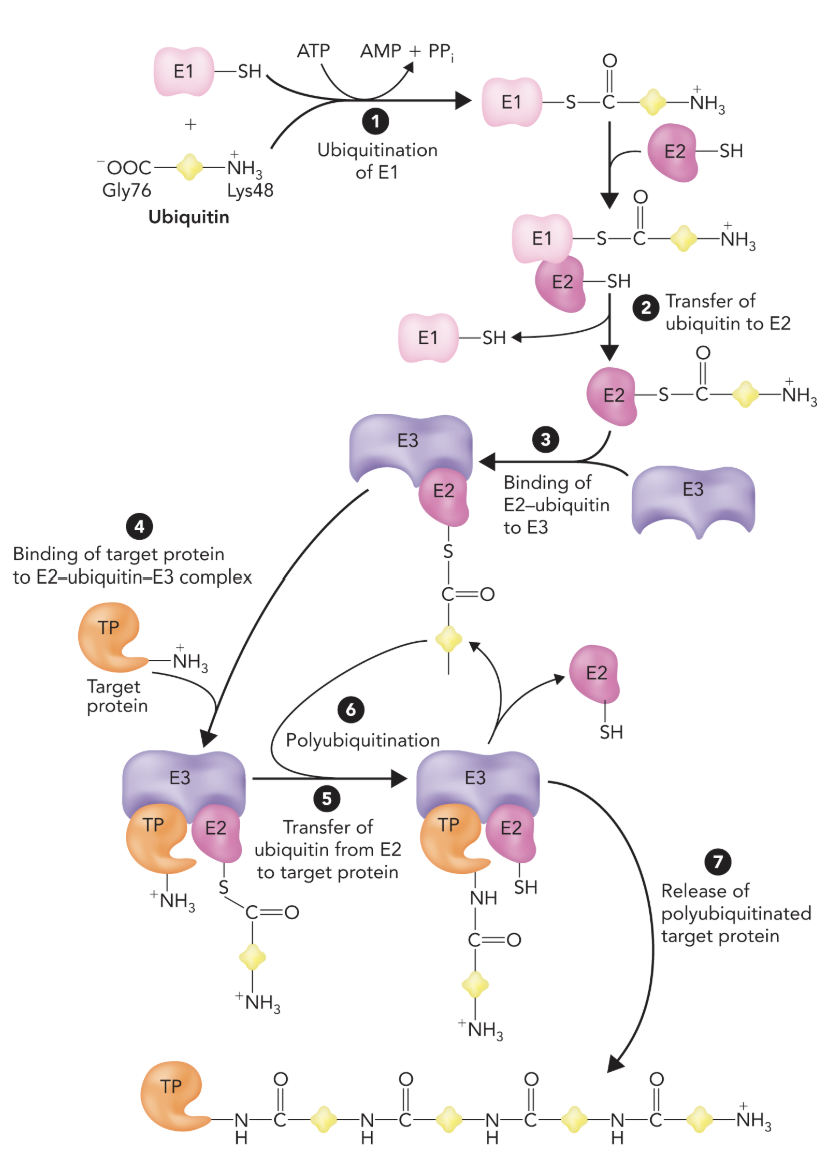
ATP-dependent proteasome pathway for proteins tagged with ubiquitin.
ubiquitin tetramer tag makes it selective
19S is the regulatory subunit that only allows ubiquitinated proteins to enter and starts the unfolding
20S subunit is the core catalytic subunit
Targets are marked for degradation by ubiquitin, involving a series of enzymes (E1, E2, and E3).
Ubiquitin-Proteasome Pathway:
The Lys attached the ubiquitin is ~76 amino acids long
The binding site for E2 on E3 the E2 must have a ubiquitin to attach
Once E2 attaches the E3 to the target protein then it is released and that process is done 4 times
E1 only 2 genes code, E2 ~30 genes code, E3 ~500 genes code; Different expressions of E3 and regulates protein degradation
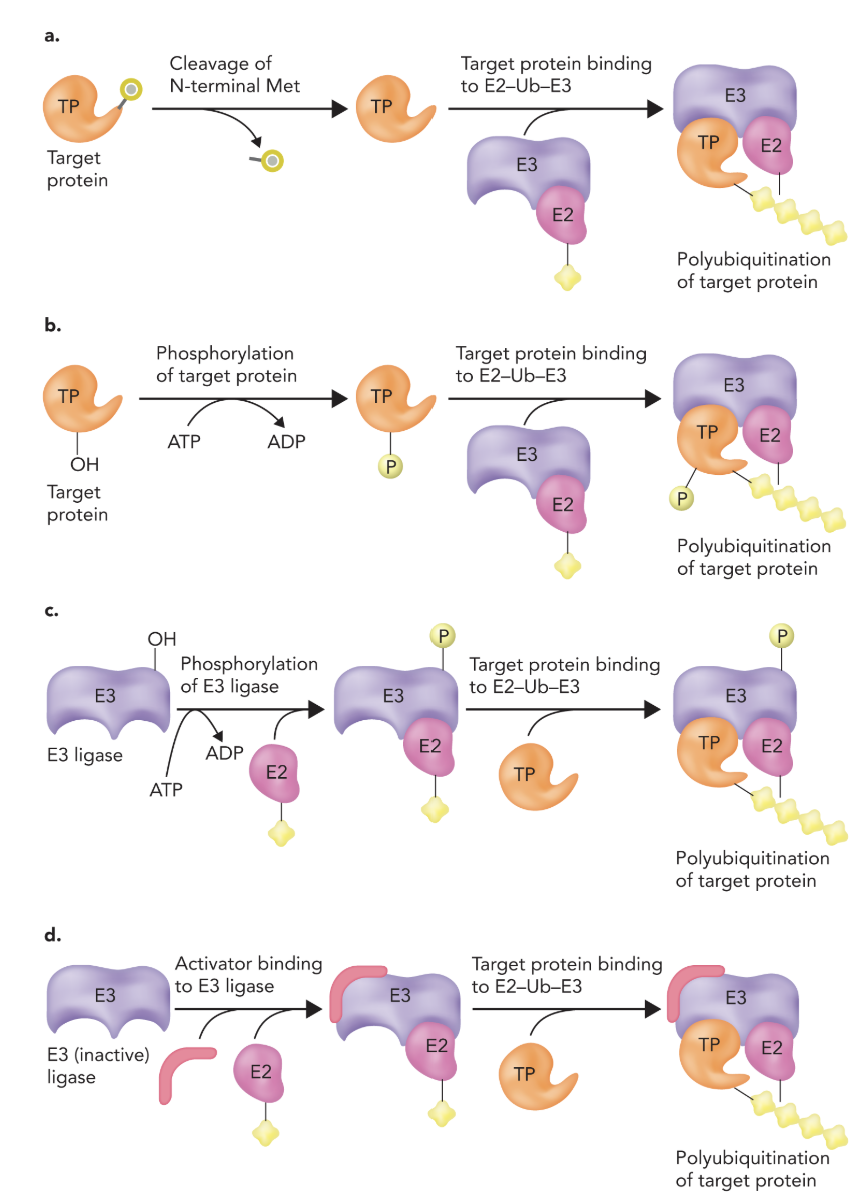
4 ways that a protein is targeted for ubiquitination
1.) N-terminal methionine cleaved
2.) Hydroxyl phosphorylation
3.) Regulate the actual enzymes in the pathway
4.) Modifying E3 (C and D; D is allosteric)
The Urea Cycle
Converts excess nitrogen from deaminated amino acids into urea, which is excreted, and utilizes intermediates like NH₄⁺, CO₂, and aspartate.
Key reactions:
Formation of carbamoyl phosphate
Cycle includes ornithine and arginine which eventually lead to urea production.
Regulated by the availability of substrates and the need for nitrogen disposal.
17.3 Amino Acid Biosynthesis
Amino acids are synthesized from three main pathways: glycolysis, pentose phosphate pathway, and the citrate cycle.
Essential vs Nonessential Amino Acids:
Essential amino acids must be acquired from diet whereas nonessential can be synthesized.
17.4 Biosynthesis of Amino Acid Derivatives
Important derivatives include heme, nucleotides, and various signaling molecules.
Heme is synthesized from the precursor glycine and succinyl-CoA and is important for respiratory proteins.
The metabolism of tyrosine leads to neurotransmitters like dopamine and hormones such as epinephrine.
Alkaptonuria and Phenylketonuria
Alkaptonuria: Caused by a defect in homogentisate-1,2-dioxygenase affecting tyrosine metabolism.
Phenylketonuria (PKU): A deficiency in phenylalanine hydroxylase, leading to toxic accumulation of phenylalanine and metabolic dysfunction.
Summary Points
Understand the interconnectedness of amino acid metabolism, urea cycle, and the nitrogen cycle.
Study the key enzymes involved in synthesis and degradation processes.
Familiarize yourself with the physiological and pathological implications of amino acid metabolism, including genetic disorders.