L5 _Membranes
Transport Across Membranes
Membrane Transport Goals
Understand the types of molecules that can easily cross the phospholipid bilayer compared to those that require more energy-intensive active transport methods.
Familiarize with various transport proteins, highlighting their structural importance and functional roles in cellular transport.
Analyze experiments demonstrating membrane protein characteristics, elucidating how these proteins facilitate selective transport.
Compare the properties of channels (which allow rapid ion flow) vs. active transporters (which move molecules against their concentrations), with an emphasis on energy usage and efficiency.
Explain the implications of membrane transporter disruptions, including how they can lead to diseases or altered cellular behaviors.
Identify key transporters and ions relevant for maintaining resting membrane potentials crucial for action potentials, which are essential for nerve signaling.
Major Components of Cellular Membranes
Structure: Cellular membranes are composed of a lipid bilayer made from phospholipids (glycerol backbone, hydrophobic fatty acid tails), sphingosine, and a diverse array of integral and peripheral proteins that contribute to fluidity and functionality.
Membrane Proteins
Types of Membrane Proteins:
Integral Proteins: These proteins span the lipid bilayer, including regions known as transmembrane domains that are typically hydrophobic, allowing them to embed within the lipid bilayer.
Lipid-anchored Proteins: These attach to the membrane via lipid tails either through covalent bonds or affinity for the lipid environment, anchoring them in place.
Peripheral Membrane Proteins: These are loosely attached to the membrane surface and can dissociate easily, often involved in signaling pathways or structural roles.
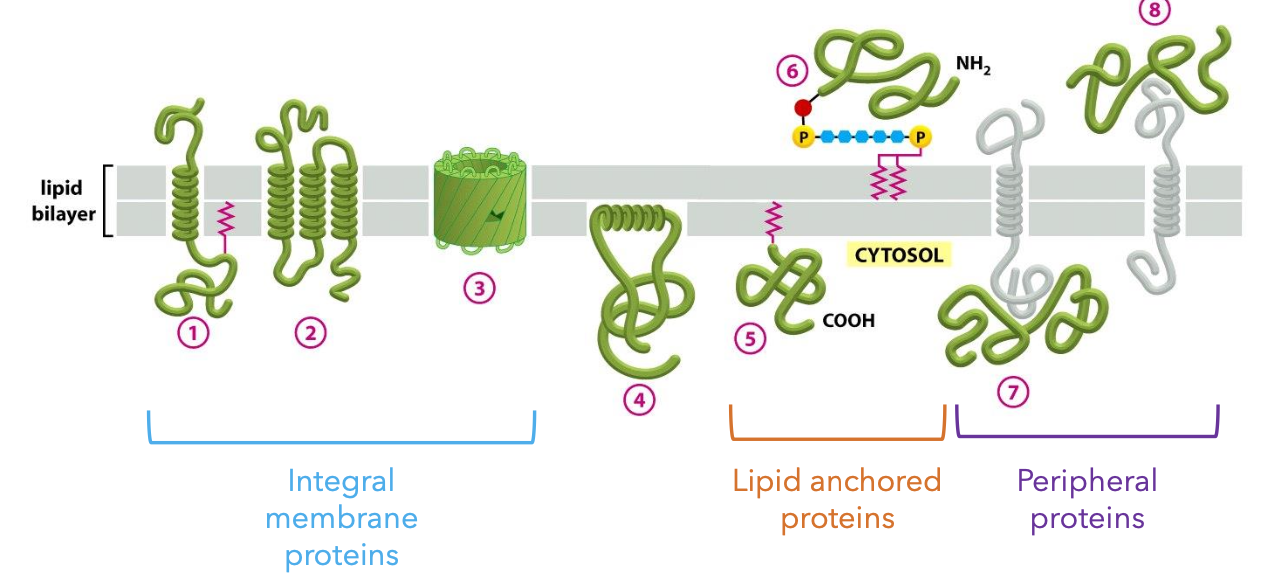
Transmembrane Domains
Example: Glycophorin A, an integral protein, features a distinctive sequence rich in glycine (Gly-X-X-X-Gly) that is crucial for its function.
Hydropathy Plot: This analytical tool is utilized to map and predict transmembrane domains based on the hydrophobic and hydrophilic characteristics of amino acid sequences.
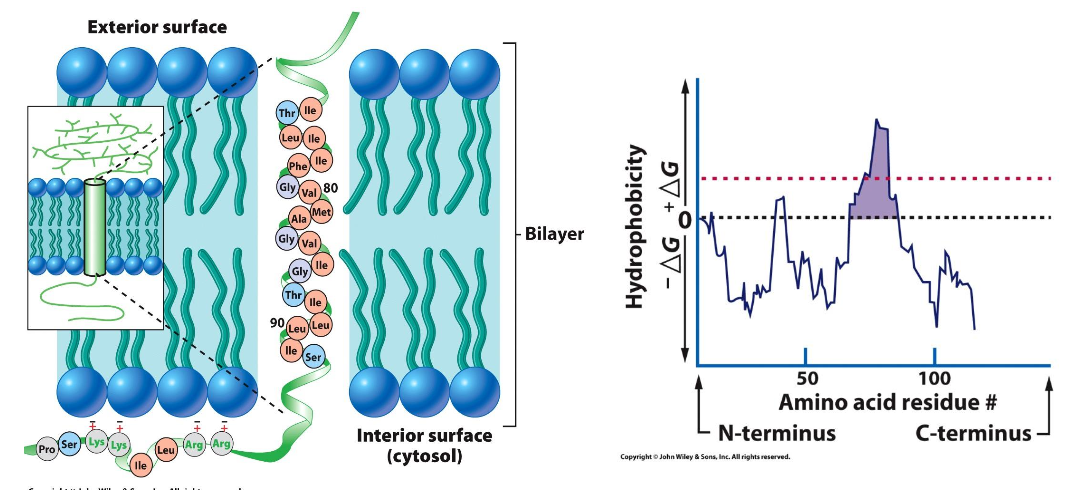
Hydrophobicity Plots
Used to generate predictions about protein topology, helping in understanding how proteins interact with the lipid bilayer and their functional placements.
Checking Topology
Various techniques such as SDS-PAGE are employed to analyze protein structure in relation to their interaction with membranes. This can illustrate how proteins assemble and function in cell membranes.
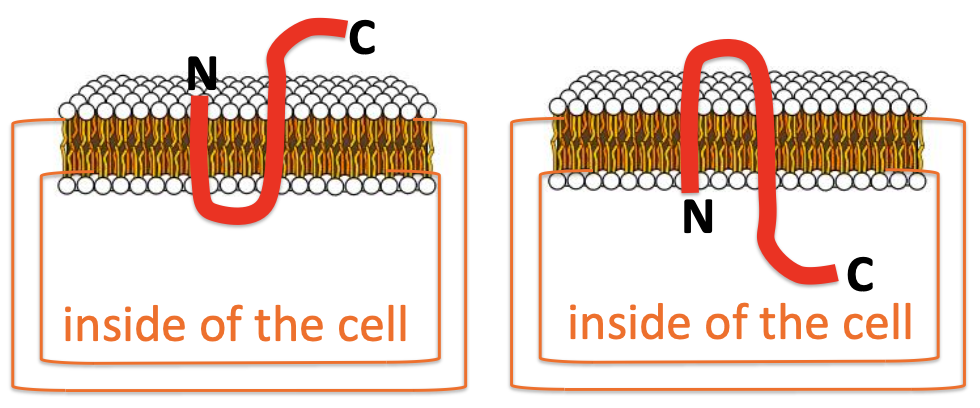
Trypsin Experiment for Membrane Topology
This experiment helps delineate external versus internal segments of membrane proteins. Trypsin, a proteolytic enzyme, cleaves external regions while transmembrane parts remain intact, facilitating localization studies.
trypsin is an enzyme that breaks proteins into very small pieces, too small. Trypsin cannot get into cell - thus transmembrane and internal proteins are protected.
Permeability of Membranes
Permeable Molecules: Smalls, nonpolar molecules and gases, such as oxygen (O2), diffuse through membranes effortlessly due to their size and polarity.
Not Permeable: Large polar molecules (e.g., glucose, sucrose) and ions cannot pass freely and necessitate specialized transport mechanisms or proteins to facilitate their movement.
Selectively Permeable: Membranes selectively allow certain molecules to pass through lipid bilayers, maintaining the internal environment of cells.
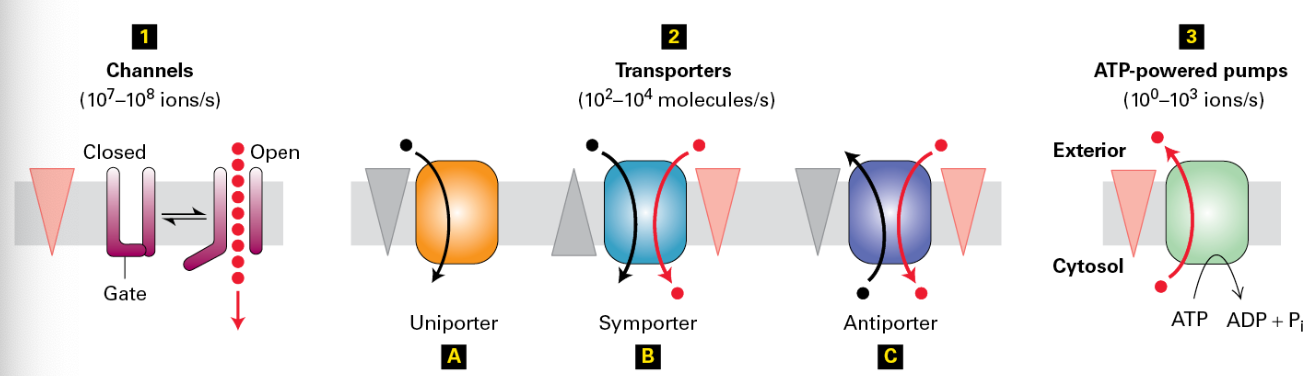
Types of Transporters
Channels: Facilitate the rapid flow of ions across membranes, capable of moving up to 10^7 - 10^8 ions per second, crucial for cellular excitability.
Transporters: Move substances through membranes at a significantly slower rate (10^2 - 10^4 molecules per second), often working through conformational changes.
ATP-Powered Pumps: Such transport mechanisms function at lower capacities but are essential for active processes, moving ions against their gradient at rates of approximately 10^3 - 10^0 ions per second.
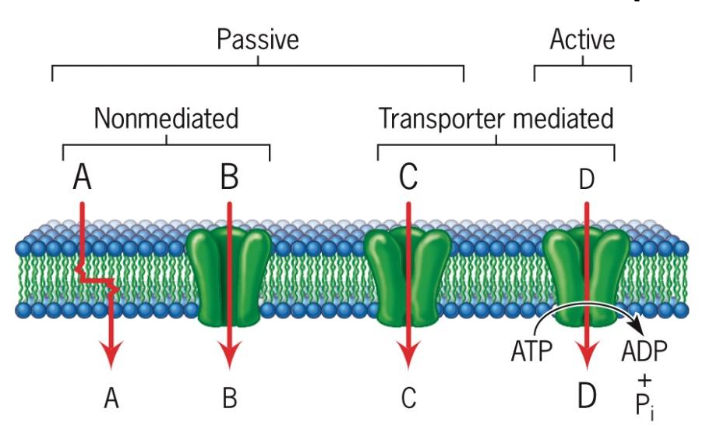
Passive vs. Active Transport
Passive Transport: This process involves the movement of substances from areas of higher concentration to lower concentration, occurring without energy input (e.g., simple diffusion, facilitated diffusion).
Active Transport: This mechanism requires energy, often supplied by ATP, to move substances against their concentration gradients, enabling cells to maintain homeostasis and internal concentrations despite external changes. Low concentration to high concentration.
Energetics of Solute Movement
The thermodynamics of solute transport can be described through the change in free energy (ΔG):
For uncharged substances, ΔG = RT ln (Ci/Co).
A negative ΔG value favors movement into the cell, while a positive ΔG indicates the need for energy to occur.
The free energy change that occurs when a solute diffuses across a membrane is dependent on the difference in concentration on the two sides of the membrane.
Facilitated Diffusion of Water and Glucose
Osmosis: Refers to the movement of water molecules from regions of low solute concentration to those of high concentration, influencing cell volume and turgor.
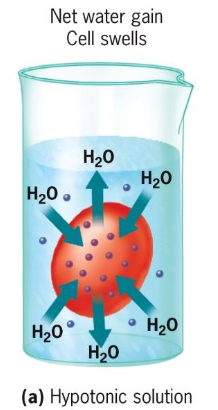
Aquaporins: H2O enters/exits cells through specialized pores. Water molecules pass through one by one very quickly! Channel wall is positively charges and binds to negatively charged O; thereby disrupting H bonds that link H2O molecules together.
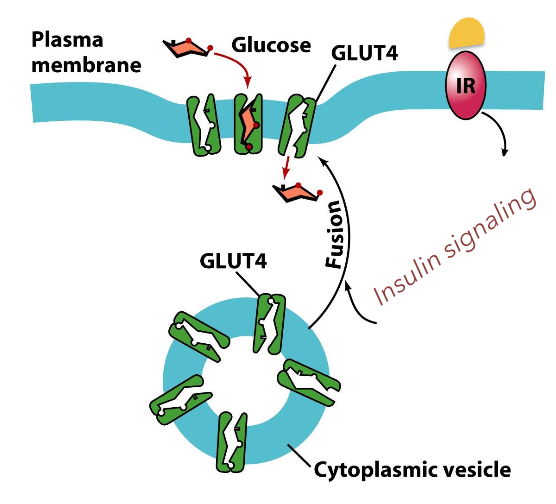
Facilitated diffusion of glucose through membranes via transporter.
diffusing substance binds selctively to a membrane spanning protein: facilitative transporter. Polar solutes and can go in both directions deterined by [gradient].
Most cells contain a glucose transporter that facilitates the diffusion of glucose from the blood stream into the cell to be used for energy.
Glucose Transporters: These transporters enable glucose to enter cells, which is vital for cellular respiration and energy production, showcasing the importance of mediating glucose dynamics in metabolism.
glucose uptake is controlled in part by regulation of its transporter on the cell surface.
insulin: hormone produced by endocrine cells of the pancreas. it maintains blood sugar levels. At low insulin levels few transporters are on the cell surface. Insulin stimulates the exocytosis of the glucose transporters.
Ion Channel Functionality
Importance of Ions: Ions such as Na+, K+, Ca2+, and Cl- cant diffuse through lipid bilayers. These are necessary for nerve impulses, muscle contraction, etc. Most ion channels are selective for specific ions and are gated.
Ion Passage: Ions traverse membranes via selective channels that respond to their concentration gradients, helping to establish resting potentials necessary for cellular response.
For an electrolyte to move across the membrane, 2 gradients must be considered.
chemical gradient determined by the concentration difference of the substance on the two sides of the membrane
the electro-potential gradient determined by the charge difference between the two sides of the membrane.
Types of Ion Channels
Voltage-Gated Channels: open based on differences in ionic charge between the inside and outside of the cell (membrane potential)
Ligand-Gated Channels: Open upon binding specific molecules (ligands), binding to the channel (conformational change). Ligands can bind to either inside or outside of membrane.
Mechanosensory Channels: These respond to physical changes in the membrane, such as tension or stress, linking mechanical stimuli to electrical signals in cells.
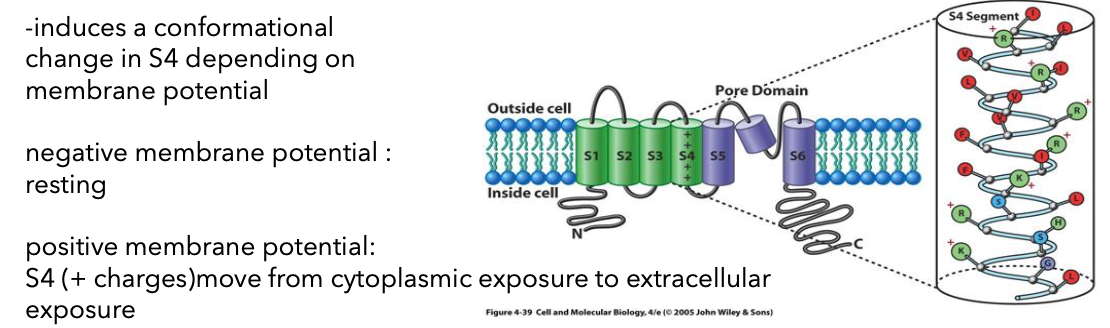
K+ Channels in Bacteria and Eukaryotes
hydrophobic alpha helices span membrane (integral membrane protein) —> tetramer
“selectivity filter” only permits k+ ions to bind
K+ is normally hydrated (O); filter provides Os (electronegative); only K+ ions fit into the selectivity filter
These channels possess selectivity filters that allow only potassium ions (K+) to traverse, which is essential for maintaining cellular potential and excitability.
In Eukaryotes they have 2 domains:
pore domain: channel, selectivity filter, and the gate (M1-
M2 helices ~ S5-S6 helices)
voltage sensor domain: sense the voltage across the membrane
Channelopathies
These genetic diseases are linked to dysfunctions in ion channels, leading to various health issues, including familial hemiplegic migraine and periodic paralysis, highlighting the clinical significance of understanding ion channel mechanics.
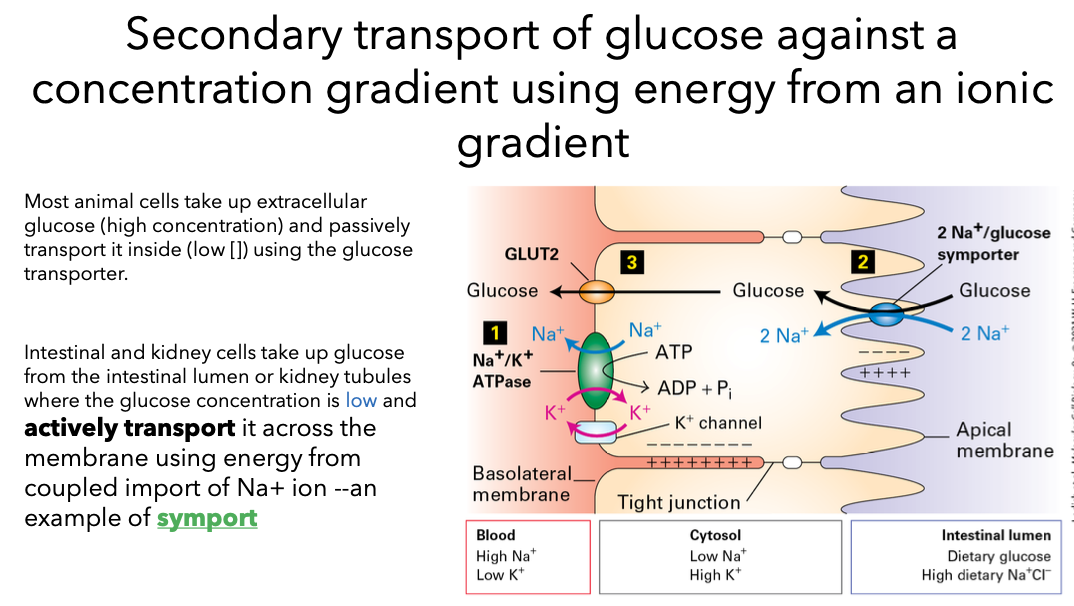
Active Transport Pumps
Active transport pumps, such as the Na+/K+ pump, are critical for maintaining concentration gradients across membranes, essential for cellular homeostasis and electrical signaling.
Mechanism: This involves a cycle of binding ions, phosphorylation (usually by ATP), and conformational changes that transport ions against their gradients.
Pumps mediate active transport - drive a given ion in only one direction. Pumps are critical:
maintain low pH inside lysosomes
maintain low pH inside the stomach
maintain electrochemical gradient for neuronal communication
Active transport: pumps move substances against a concentration gradient
Critical elements of Na+/K+ Pump
Pumps ions AGAINST the concentration gradient
Hydrolysis of ATP is required (“P-type” pump: hydrolysis of ATP results in phosphorylation of the transport protein)
Transport protein must have a higher binding affinity for Na+ inside the cell and a lower binding affinity for Na+ outside of the cell
Transport protein must have a higher binding affinity for K+ outside of the cell and a lower binding affinity for K+ inside the cell
Different affinities are achieved by phosphorylating the transport protein
Pump is necessary to maintain steep gradient required for nerve and muscle cell activation.
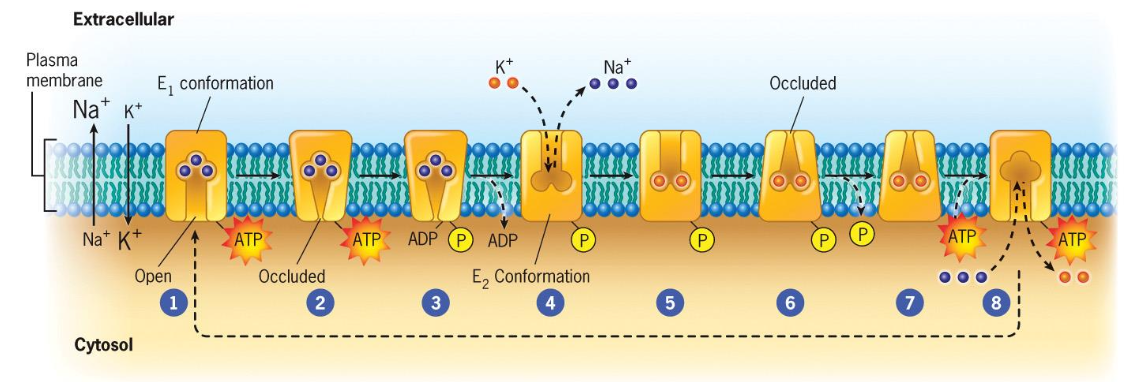
This pump creates the big difference in the concentrations of Na+ and K+ inside vs outside the cell.
Na+ binds to the transport protein on the inside of the cell with high affinity.
A gate within the protein closes Na+ ions can no longer flow back into the cytosol
ATP is hydrolyzed and the released P binds to the transport protein
binding of P changes the transporters configuration and affinity for Na+, Na+ is released to the outside of the membrane
K+ bind to the pump
Closes another gate within the protein preventing the back flow of the k+ ions into the extracellular space
dephosphorylation and ATP binding induces the return of the protein to the original conformation
binding site open to the internal surface of the membrane and loses affinity for K+. k+ released into the cell.
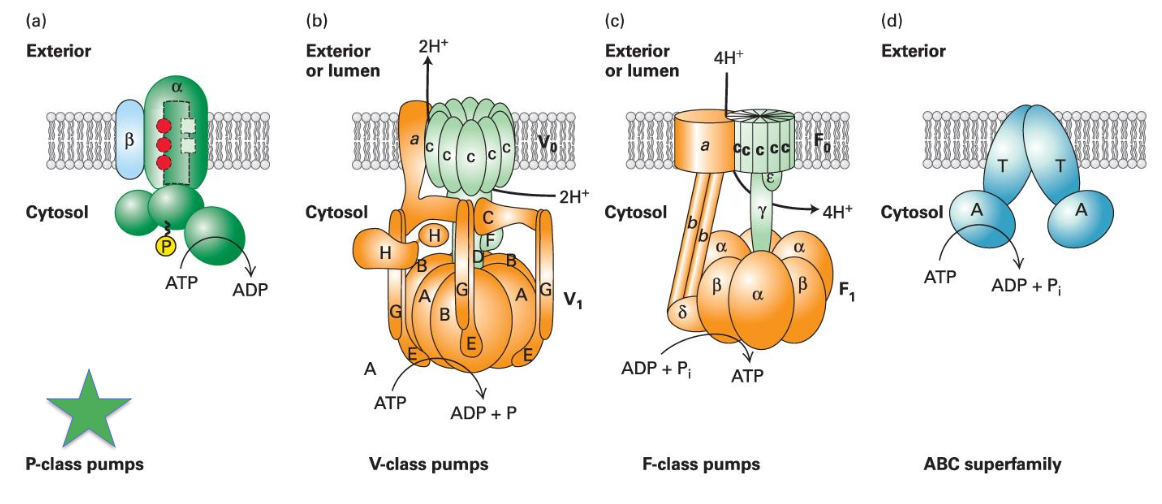
Many ATP powered pumps
Na+/K+ pump is only in animal cells
H+/K+ pump in the stomach (pumps acid into the stomach; pump translocates to plasma membrane after eating)
H+ proton pump in plants is important for import of solutes and control of pH
ABC (ATP-binding cassette) superfamily of pumps
present in bacteria through mammals
pump ions, sugars, peptides, polysaccharides, proteins!
Defects in pumps, transporters, or channels often lead to disease