Radioactivity
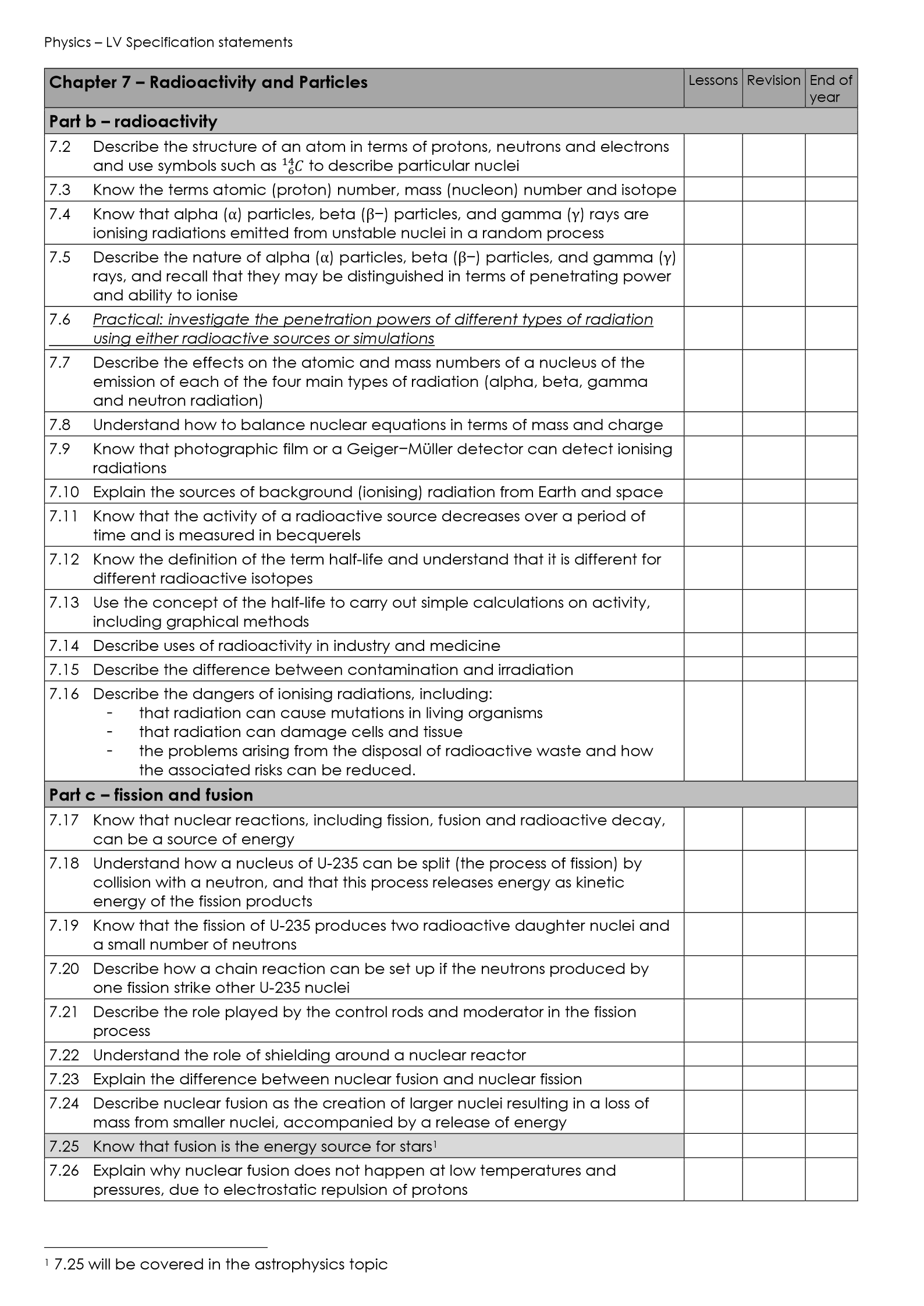
Structure of an Atom
Atoms are the basic units of matter, made up of three subatomic particles:
Protons
Found in the nucleus
Positively charged
Determine the atomic number and element’s identity
Neutrons
Also in the nucleus
No charge
Help stabilize the nucleus and add to atomic mass
Electrons
Move in energy levels (or shells) around the nucleus
Negatively charged
Their arrangement affects the element’s chemical behaviour
Isotopes are variations of the same element with the same number of protons but different numbers of neutrons, affecting their mass but not their chemical properties
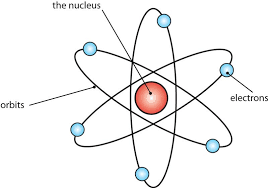
Atomic Number, Mass Number, and Isotope
Atomic Number (Z): Indicates the number of protons in the nucleus. For example, Carbon has an atomic number of 6.
Mass Number (A): The total number of protons and neutrons in the nucleus. An isotope’s mass number can vary (e.g., Carbon-12 and Carbon-14).
Isotopes: Different isotopes of an element have identical chemical behaviour but may have different physical properties, such as stability. Radioactive isotopes (radioisotopes) undergo decay and emit radiation.
Alpha, Beta, and Gamma Radiation
Alpha (α) particles:
Consists of 2 protons and 2 neutrons (like a helium nucleus).
They are heavy, positively charged, and have low penetration power (can be stopped by paper or skin).
High ionizing power means they can cause significant damage if inhaled or ingested.
Beta (β) particles:
High-speed electrons (β-) or positrons (β+).
Lighter and more penetrating than alpha particles, they can pass through paper but are stopped by materials like plastic or aluminium.
Moderate ionizing power; can penetrate the skin, posing a risk of internal damage.
Gamma (γ) rays:
Electromagnetic waves with no mass or charge.
Highly penetrating, requiring dense materials like lead or thick concrete for shielding.
Least ionizing but can still cause damage by interacting with cells deep within the body.
Table: Properties of Radiations
Radiation Type | Charge | Penetration Power | Ionizing Power | Shielding Material |
Alpha (α) | +2 | Low (stopped by paper) | High | Paper, skin |
Beta (β) | -1/+1 | Medium (stopped by plastic) | Medium | Plastic, aluminium |
Gamma (γ) | 0 | High (stopped by lead) | Low | Lead, thick concrete |
Nature and Differentiation of Radiations
Alpha radiation is heavy and highly ionizing but lacks penetration power. It poses a risk primarily if radioactive material is ingested or inhaled.
Beta radiation has more penetration power and can travel through soft tissue, causing potential internal damage if shielding (like clothing or aluminium) is not used.
Gamma rays, being highly penetrating, can travel through the body and other materials, making them hazardous without proper shielding (lead or concrete).
Practical Investigation
To investigate radiation types, a Geiger-Müller counter is used to detect levels of radiation:
Alpha radiation: Easily blocked by paper or a few centimetres of air; detected when the source is unshielded.
Beta radiation: Can penetrate paper but is stopped by a few millimetres of aluminium or plastic.
Gamma radiation: Only reduced significantly by dense materials like lead; requires careful handling due to its penetration capability.
Understanding penetration helps in designing appropriate safety measures for radiation use and exposure.
Extended explanation:
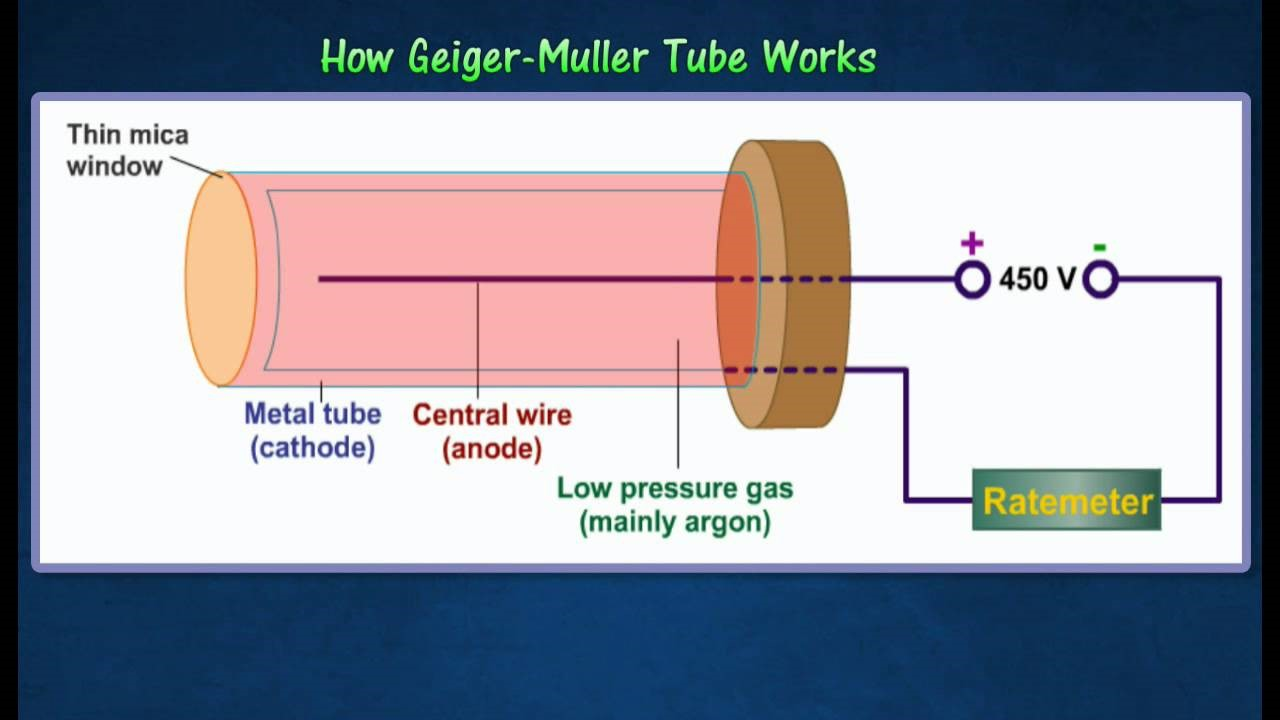
To determine the level of radioactivity of alpha, beta, or gamma radiation, a Geiger-Müller (GM) tube is commonly used. The GM tube detects and measures ionizing radiation by counting the number of ionized particles produced when radiation passes through the tube. Here's how the method works:
Measurement procedure: Background Measurement: Measure background radiation levels first, without any radioactive sources, to establish a baseline.
Sample Measurement: Place the radioactive sample (alpha, beta, or gamma emitter) close to the GM tube.
For alpha radiation: Position the sample very close (a few centimetres) because alpha particles have low penetration power and are easily stopped by air.
For beta radiation: Maintain a short distance (a few centimetres to a few meters) as beta particles have more penetration power but can still be absorbed by solid materials.
For gamma radiation: Position the GM tube further away, as gamma rays are highly penetrating and can travel through several meters of air or dense materials.
Results:
Alpha Radiation:
A significant increase in CPS/CPM when the source is very close indicates alpha radiation.
The counts will drop sharply if the source is moved even slightly away due to the limited range of alpha particles.
Beta Radiation:
If CPS/CPM increases and remains higher even when the source is a few centimetres or meters away, it suggests beta radiation.
Counts will also drop when a thin material like plastic or aluminium is placed between the tube and the source.
Gamma Radiation:
A consistent increase in counts, even with greater distance and shielding material (e.g., lead), suggests gamma radiation due to its high penetration power
Recording Data: Measure the CPS or CPM over a set period, usually a few minutes, to get a reliable average reading
Effects on Atomic and Mass Numbers
Alpha decay: Reduces the atomic number by 2 and the mass number by 4 as a helium nucleus is emitted:
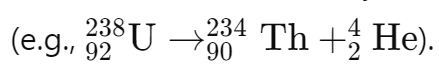
Beta decay: Increases the atomic number by 1 when a neutron transforms into a proton and emits an electron (β-):
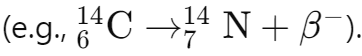
Gamma emission: No change in atomic or mass numbers, as gamma rays release excess energy without altering the nucleus's structure.
Balancing Nuclear Equations
Nuclear equations must conserve both mass number (A) and atomic number (Z). This ensures that the reactants and products match, reflecting the conservation laws governing nuclear reactions.
Detection of Ionizing Radiation
Photographic film: Darkens upon exposure to radiation; used in film badges for monitoring exposure.
Geiger-Müller counter: Detects radiation particles and produces an audible click for each event, measuring the intensity of ionizing radiation.
Background Radiation
Natural sources include:
Cosmic rays: High-energy particles from space.
Radon gas: Emanates from rocks and soil.
Artificial sources include medical procedures like X-rays and nuclear energy production.
Background radiation levels vary with location (e.g., higher in areas with granite rocks).
Radioactive Decay Over Time
Radioactive substances decay at a characteristic rate, measured in becquerels (Bq). A becquerel indicates one decay event per second.
The rate of decay, known as the activity, decreases over time, forming the basis of decay curves.
Half-Life
The half-life of a radioactive isotope is the length of time it takes for half of its nuclei to decay. Each isotope has a unique half-life (e.g., Carbon-14: 5730 years).
This concept is crucial in dating ancient artifacts, medical treatments, and managing radioactive waste.
Finding half life from a graph:
Pick a pair of values on the axis where one is half the other
Read the x-axis values for each (draw interpolation lines)
Find the difference between the x-axis values. That is the half life
Repeat for another pair of values to find the mean
Calculations Using Half-Life
N is the remaining quantity, N0 is the initial amount, and n is the number of half-lives elapsed.
This formula helps predict how quickly a sample becomes safe or less hazardous.

Radioactivity in Industry and Medicine
In medicine, gamma rays sterilize equipment and are used in imaging techniques (e.g., PET scans).
In industry, radioisotopes check material thickness or inspect welds for safety and integrity.
Contamination vs. Irradiation
Contamination: Occurs when radioactive materials are present on or inside objects, leading to ongoing exposure.
Irradiation: Involves exposure to radiation without the material being present. This is temporary and stops once the source is removed.
Dangers and Protection
Mutations and Cancer: Ionizing radiation can damage DNA, leading to mutations and increasing cancer risk.
Cell Damage: Radiation exposure can destroy cells and tissues, affecting organs and functions (e.g., radiation burns or acute radiation syndrome).
Waste Management: Proper disposal and containment of radioactive waste are critical to prevent environmental contamination.
Safety Measures: Use of shielding, minimizing exposure time, and maintaining distance are essential practices to reduce risks.
Nuclear Reactions as Energy Sources
Nuclear reactions, including fission, fusion, and radioactive decay, release energy stored in the nucleus of an atom. This energy comes from converting a small amount of mass into energy, following Einstein’s equation E=mc2
Fission: The splitting of a heavy nucleus (e.g., uranium-235) into two lighter nuclei, releasing energy, neutrons, and gamma radiation.
Fusion: The combination of two light nuclei (e.g., hydrogen isotopes) to form a heavier nucleus (e.g., helium), releasing even more energy than fission.
Fission of U-235
It can absorb a neutron, becoming unstable and splitting into two smaller nuclei (fission fragments), such as barium and krypton. This process releases:
Kinetic energy: As the fragments fly apart.
Neutrons: Which can initiate further fission reactions (chain reaction).
Gamma radiation: Emitted as the nuclei stabilize, contributing to the energy released.
Example of the reaction:
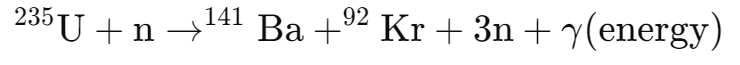
The released energy is harnessed in nuclear power plants to generate electricity.
Fission Products of U-235
The fission of uranium-235 produces:
Two radioactive daughter nuclei (fission products), which vary in size but are typically around half the mass of the original nucleus (e.g., barium and krypton).
A few additional neutrons (usually 2 or 3), which can induce further fission events if they collide with other uranium-235 nuclei.
The neutrons’ release is crucial for maintaining a chain reaction, which is controlled in reactors for energy generation or uncontrolled in nuclear weapons.
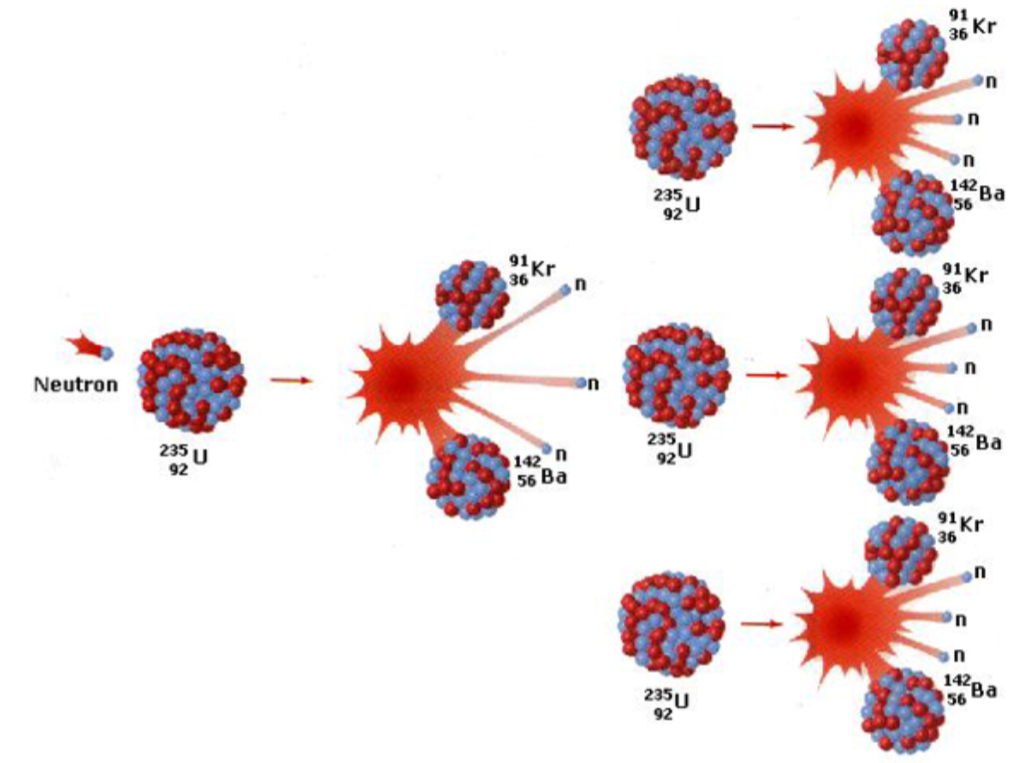
Chain Reaction in Nuclear Fission
A chain reaction occurs when the neutrons produced by the fission of one nucleus go on to trigger fission in other nuclei.
This leads to an exponential increase in reactions if not controlled.
In nuclear reactors, control rods (usually made of boron or cadmium) absorb some of the excess neutrons to regulate the reaction rate and prevent the reaction from escalating uncontrollably.
For a sustained chain reaction, the system must maintain a critical mass of the fissile material to ensure a continuous reaction.
Role of Control Rods and Moderator
Control Rods: Inserted into the reactor core to absorb neutrons and slow down the reaction. By adjusting the rods’ position, operators can control the rate of fission.
Moderator: Typically water or graphite, it slows down the fast-moving neutrons produced during fission. Slower neutrons are more likely to be captured by uranium-235 nuclei, maintaining the chain reaction at a steady rate.
Without these components, the fission reaction could become uncontrolled, leading to overheating or even a meltdown.
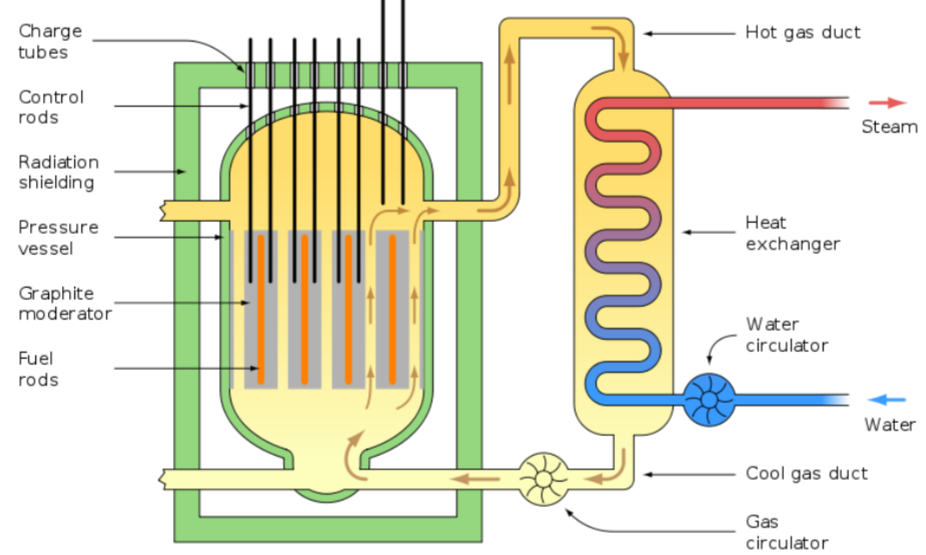
Shielding Around Nuclear Reactors
Nuclear reactors are surrounded by shielding materials, usually thick layers of concrete and lead, to block harmful radiation (gamma rays and neutrons) from escaping into the environment.
The primary shielding protects workers and the public from exposure, while the secondary containment provides an additional layer of safety, particularly in the event of an emergency or reactor malfunction.
Nuclear Fusion vs. Nuclear Fission
Fission:
Splits a heavy nucleus into smaller fragments.
Releases energy, along with neutrons and gamma radiation.
Used in nuclear reactors and atomic bombs.
Fusion:
Combines light nuclei (e.g., hydrogen isotopes) to form a heavier nucleus (e.g., helium).
Releases significantly more energy than fission and produces fewer radioactive by-products.
Occurs naturally in stars but is challenging to achieve and maintain on Earth due to the extreme conditions required.
Nuclear Fusion as Energy Creation
Fusion is the process where two light nuclei, such as deuterium (2H) and tritium (3H), combine under extreme temperature (millions of degrees Celsius) and pressure to form a heavier nucleus (e.g., helium).
The reaction releases energy:
Reaction example:
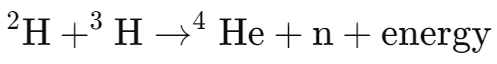
The mass lost in the reaction is converted into energy.
Fusion offers a potential source of clean energy because it produces no greenhouse gases or long-lived radioactive waste.
However, achieving the necessary conditions on Earth (e.g., using tokamak reactors) remains a technological challenge.
Why Fusion Does Not Occur at Low Temperatures and Pressures
Nuclear fusion requires extremely high temperatures (millions of degrees) because positively charged nuclei (protons) naturally repel each other due to their electrostatic repulsion.
Only at very high temperatures do nuclei gain enough kinetic energy to overcome this barrier and collide forcefully enough to fuse.
At low temperatures and pressures, the nuclei lack the energy needed to overcome this repulsion, making fusion impossible under normal conditions on Earth.
Waste management extension:
1. Site Selection and Evaluation
Choose geologically stable locations to prevent earthquakes, floods, or other natural disasters that could damage the facility.
Ensure low groundwater movement to minimize the risk of waste contamination spreading.
Evaluate population density and accessibility to minimize human exposure and provide ease of transport.
2. Waste Isolation and Containment Systems
Physical Barriers: Use multiple engineered barriers like steel canisters, concrete encasements, and specialized storage containers to isolate radioactive waste.
Buffer Materials: Add bentonite clay or other materials around waste canisters to prevent water infiltration and limit the migration of radioactive particles.
Backfill: Use materials like crushed rock to fill any voids in the repository, stabilizing the structure and further isolating the waste.
4. Ventilation and Radiation Shielding
Install systems that manage air circulation to control temperature and radiation levels.
Utilize thick concrete and lead walls around storage areas to absorb radiation and protect workers during handling and inspection processes.
5. Monitoring and Surveillance Systems
Integrate radiation detectors and monitoring devices to continuously measure radiation levels, temperature, and groundwater quality.
Establish a long-term monitoring plan, with remote sensing technology, to detect any early signs of leakage or contamination.
7. Regulatory Compliance and Safety Standards
Adhere to international and national regulations set by organizations such as the International Atomic Energy Agency (IAEA).
Regularly update practices and safety protocols based on scientific developments and risk assessments.
8. Long-term Security Measures
Implement physical security (e.g., fencing, surveillance cameras) to prevent unauthorized access to storage sites.
Develop clear signage and documentation to warn future generations about the location and dangers of the waste, ensuring long-term awareness.
Extra info:
Evaluation: Dangers of Radiation and Safety Measures:
Health Risks
Ionizing radiation from fission and radioactive decay can cause severe health effects, including:
Cancer: DNA damage from radiation exposure can result in mutations that lead to cancer. Gamma rays and beta particles are particularly concerning as they penetrate deep into body tissues.
Acute Radiation Syndrome (ARS): High doses of radiation can kill large numbers of cells, leading to symptoms like nausea, burns, and in severe cases, organ failure or death.
Genetic Mutations: Radiation can also affect reproductive cells, leading to genetic mutations in offspring.
Environmental Risks
Improper disposal of nuclear waste can contaminate water supplies, soil, and air, causing long-term environmental and health problems.
Radioactive accidents (e.g., Chernobyl, Fukushima) highlight the risks associated with fission reactors, emphasizing the need for effective safety measures and containment systems.
Safety Measures
Shielding: The use of lead, concrete, and water as barriers to block radiation.
Minimizing Exposure: Using the principles of time, distance, and shielding (TDS):
Time: Reducing the duration of exposure to radiation sources.
Distance: Keeping a safe distance from radiation sources, as intensity decreases with distance.
Shielding: Employing protective barriers.
Monitoring: Personal dosimeters measure radiation exposure for workers, ensuring that they remain within safe limits.
Nuclear Waste Management
Radioactive waste, a by-product of fission, requires long-term storage solutions (e.g., deep geological repositories) to prevent environmental contamination.
New technologies aim to reduce the volume and longevity of nuclear waste through recycling and advanced reactors that can utilize waste as fuel.