Carbohydrates and Lipids
Carbohydrates and lipids
Organic vs inorganic compounds
The main difference is that organic compounds contain the element carbon and are found in living things, however inorganic compounds don’t have carbon but it’s still important in living things for structure and functionality such as water (h20)
Organic compounds’ carbon atoms allow the formation of diverse compounds upon life because it has 4 valence electrons that want to bond with other atoms creating 4 very strong covalent bonds.
Carbon is special because it is a small atom which allow it to fit into and and form several types of structures. Additionally, it isn’t reactive so they can easily make and destroy bonds with other atoms.
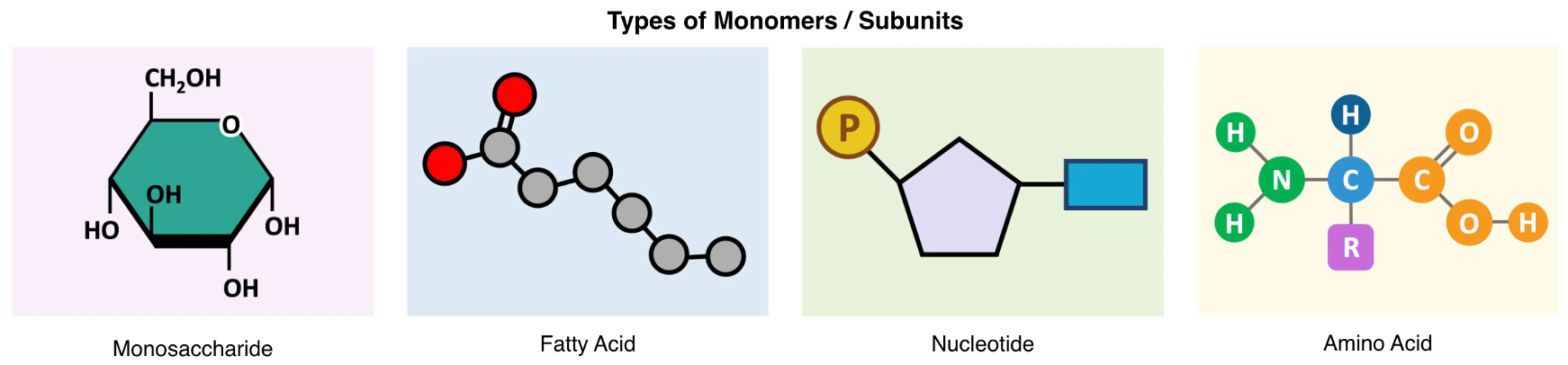
Organic compounds are typically composed of recurring subunits and are covalently joined to form polymers. For example, nucleotides (monomers) make nucleic acids (macromolecules) in turn they make DNA or RNA (polymers). Some other examples such as amino acids making up proteins, fatty acid and glycerol making lipids and monosaccharides making carbohydrates.
 This is done through a process called metabolism.
This is done through a process called metabolism.
Metabolism is the term for all the enzymes catalysed reactions in a cell or organisms and it has 2 types.
Anabolic:
Condensation reaction
Monomers put together to make macromolecules
And Catabolic:
Hydrolysis reaction
macromolecules broken down into monomers
Condensation reactions:
When a hydroxyl group on one monomer is combined with a hydrogen atom on another monomer which results in the two monomers becoming covalently bonded and a water molecule is produced as a by product.
This is seen in anabolism. For example, monosaccharide + monnosaccharide -> disaccharide + water. Further monomers can be combined to make polymers. For example, nucleotide + nucleotide + nucleotide = nucleic acid which can be further combined to make RNA or DNA.
Hydrolysis
Hydrolysis on the other hand is opposite. A water molecule is split to provide the hydroxyl and hydrogen groups to break the covalent bond between the two monomers and can be seen in catabolism for example starch - > glucose.
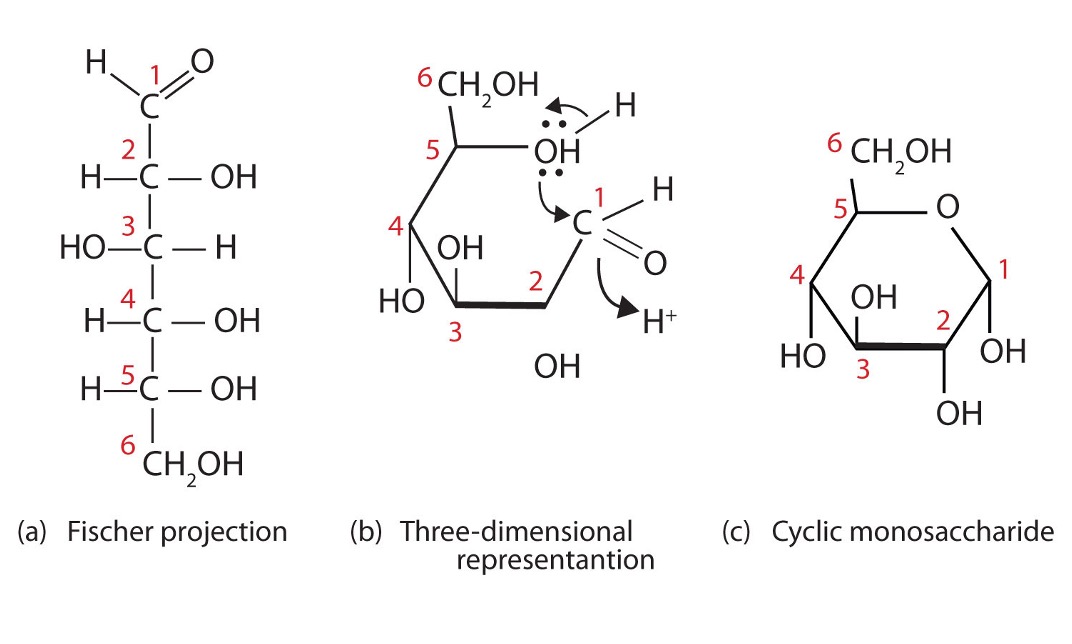
Form of a monosaccharide
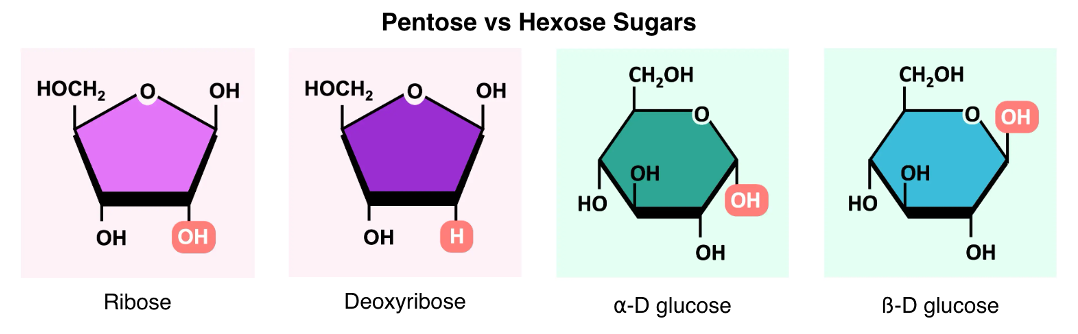
•Monosaccharides typically form ring structures as a result of a chemical reaction between functional groups at opposite ends of the molecule.A hydroxyl group (-OH) links to a carbonyl group (=O) to form a cyclic structure connected by an oxygen atom. Most monosaccharides have either 5 carbons (pentose sugars) or 6 carbons (hexose sugars). The name describes the number of carbons – not the shape (e.g. fructose is a hexose sugar but forms a pentagon). An example of a pentose sugar is ribose and deoxyribose which are found in DNA & RNA. An example of a hexose sugar is glucose – which is primarily used as a source of energy (it is digested via cell respiration to produce ATP). Glucose can exist as one of two isomers (⍺-D glucose or ß-D glucose) depending on the orientation of the 1’-OH group. •Polymers of ⍺-glucose are used in energy storage; glycogen is used in animals and starch is used in plants.
 Function of monosaccharides
Function of monosaccharides
The main function of monosaccharides is as a source of energy. Monosaccharides are broken down to produce large quantities of biological energy (ATP) via cellular respiration. An example of this is glucose. Glucose is the most common monosaccharide to be used as an energy source due to its various chemical properties:
•Solubility: Glucose is a polar molecule (due to –OH groups) and so will dissolve in water (it is hydrophilic)
•Stability: Glucose is a very stable molecule as cyclic structures are generally more energetically favourable than straight chains
•Transport: Because glucose is soluble and stable, it is easier to transport within aqueous solutions (like blood or cytosol)
•Potential Energy: Glucose has many high energy electrons (between C–C and C–H bonds) which can be released via oxidation
•ATP Yield: Glucose can by oxidised to produce a large yield of ATP via aerobic cell respiration
However, polysaccharides can exist as energy storage compounds too. There are 3 main forms that carbohydrate polysaccharides exist in:
Cellulose
Glycogen
Starch
Glucose monomers can be added or removed (by condensation or hydrolysis reactions) to build or mobilise these energy stores.
Glycosidic linkage
A glycosidic linkage is a covalent bond that connects a carbohydrate molecule to another group (note: can be another carbohydrate or sometimes other molecules). In both glycogen ( alpha glucose for animals) and starch (alpha glucose for plants) , the ⍺-glucose monomers are connected via 1’ – 4’ glycosidic linkages to form helical structures
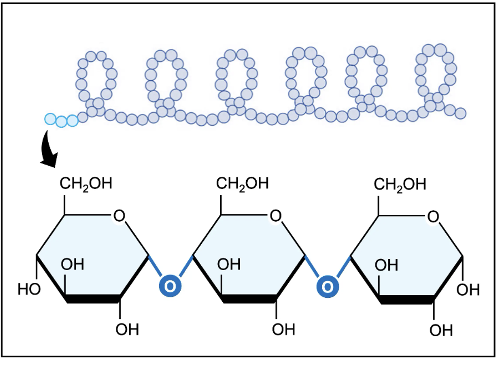 Starch, is used as an energy source in plants and exist as linear strands or branched due to the presence of additional 1’-6’ linkages. Branching can cause the polysaccharide to adopt more compact structure, but their large molecular size renders them insoluble in water. Therefore, glycogen and starch are efficient storage molecules that are not suitable from transport within aqueous solutions. However, the carbs can be digested to release monomers or dimer for transport.
Starch, is used as an energy source in plants and exist as linear strands or branched due to the presence of additional 1’-6’ linkages. Branching can cause the polysaccharide to adopt more compact structure, but their large molecular size renders them insoluble in water. Therefore, glycogen and starch are efficient storage molecules that are not suitable from transport within aqueous solutions. However, the carbs can be digested to release monomers or dimer for transport.
Glycogen, an energy source for animals is more branches than starch as ig has more frequent 1’-6’ linkages; having it to be every 10 subunits, and starch only 20
Cellulose
Cellulose is a linear molecule that is made of beta glucose subunits (1’-4’ arrangement) and the beta glucose exist in alternating arrangements. The alternating subunits allows cellulose to form straight linear chains that can be grouped in bundles and cross-linked with hydrogen bonds These cross-linked bundles function to increase the structural integrity and mechanical stability of the polymer
Beta glucose is used for structural reasons, which is why it can be found in the cell walls of plants, otherwise known as Cellulose. Because it is made of beta glucose, it’s actually indigestible for most animals because they lack the enzyme required to break it down.
Role of glycoproteins in cell-cell recognition
Carbohydrates are attached to the protein in the cell membrane and it is called glycoproteins. Glycoproteins are found in many places in the body, but in the cell membrane their key function is cell-cell recognition.
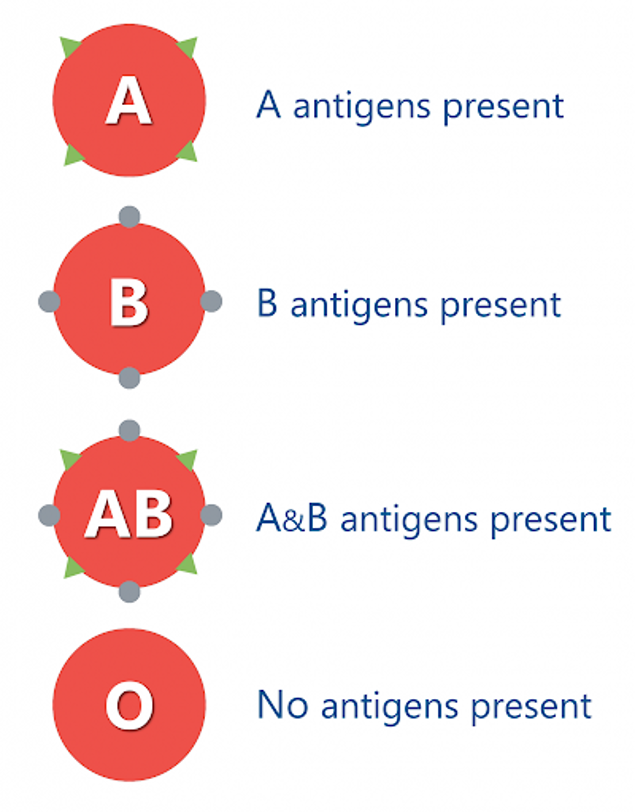
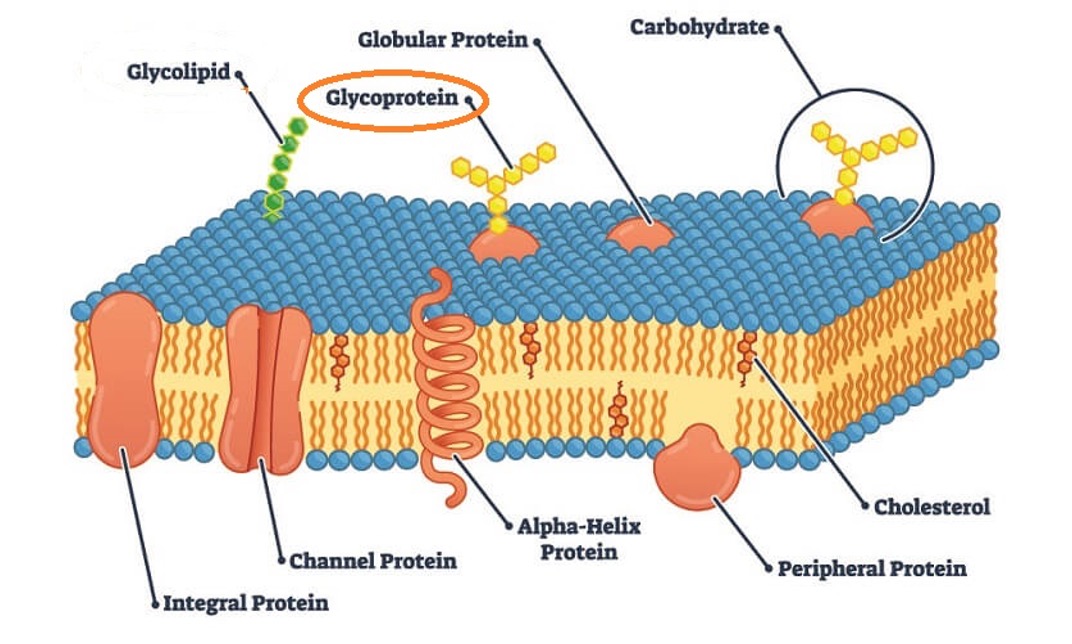 The glycoprotein on one cell can be recognised by the receptor on the surface of another cell and is important because it allows the body to differentiate between itself and foreign cells such as viruses. An example of this is ABO antigens, red blood cells have glycoproteins on their plasma membranes that distinguish ABO blood types.
The glycoprotein on one cell can be recognised by the receptor on the surface of another cell and is important because it allows the body to differentiate between itself and foreign cells such as viruses. An example of this is ABO antigens, red blood cells have glycoproteins on their plasma membranes that distinguish ABO blood types.
 Lipids
Lipids
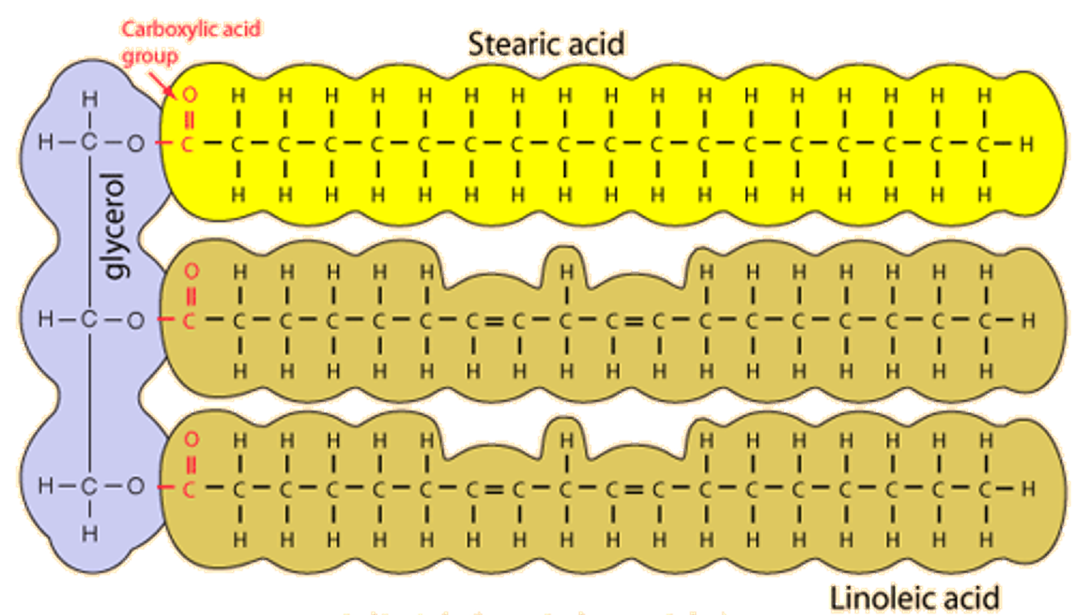
Lipids are fats, oils, waxes and steroids and triglycerides can be joined with a phosphate to form a phospholipid (recall the phospholipid bilayer). Joined by Ester bonds, their general structure is compounds of glycerol (same structure for all) and fatty acids; found in many forms.
 Some lipids have a hydrocarbon ring instead of a chain though. These hydrocarbons are non polar (lack charge) and will not dissolve in polar substances such as water which is why it’s considered be hydrophobic.
Some lipids have a hydrocarbon ring instead of a chain though. These hydrocarbons are non polar (lack charge) and will not dissolve in polar substances such as water which is why it’s considered be hydrophobic.
Because of these properties, many good things happen from this.
Waxes are used to prevent water loss from leaves while birds coat their feathers with oil to render them waterproof.
Phospholipids provide a structural framework for cells by forming spontaneous membranes in aqueous solutions.
Lipids in foods help the body to absorb certain fat-soluble micronutrients, including vitamins A and D.
However, the hydrophobic properties of lipids make them difficult to transport around the body.
Digested fats are packaged within a protein coat to become water soluble lipoproteins
Steroid hormones are bound to carrier proteins (such as albumin) to facilitate their movement through the bloodstream
Certain lipids have polar components, becoming amphipathic (having both hydrophobic and hydrophilic regions)
Phospholipids possess a polar phosphate head, cholesterol has a polar hydroxyl group and glycolipids can have polar carbohydrate chains
These amphipathic molecules all have a limited capacity to interact with water, helping them to function in maintaining membrane integrity
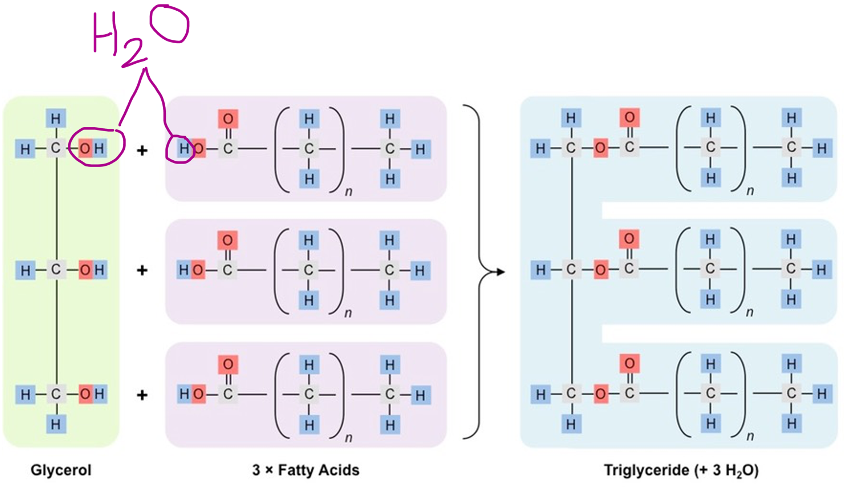
Formation of triglycerides and phospholipids by condensation reactions.
Unlike the other macromolecules, technically lipids are not polymers because they don’t have recurring subunits or monomers. But some common ones have glycerol and fatty acid chains, not counting steroids. The fatty acids can be linked to they hydroxyl group of alcohols via condensation reaction to produce an ester linkage, a bond between an alcohol and an organic acid.
 Difference between saturated, monounsaturated and polyunsaturated fatty acids
Difference between saturated, monounsaturated and polyunsaturated fatty acids
•Saturated = no double bonds between carbons and maximum H atoms present (saturated with hydrogens, cannot have more hydrogens)
•Monounsaturated = 1 double bond between carbons (could hold more hydrogens)
•Polyunsaturated = 2 or more double bonds between carbons (could hold more hydrogens)
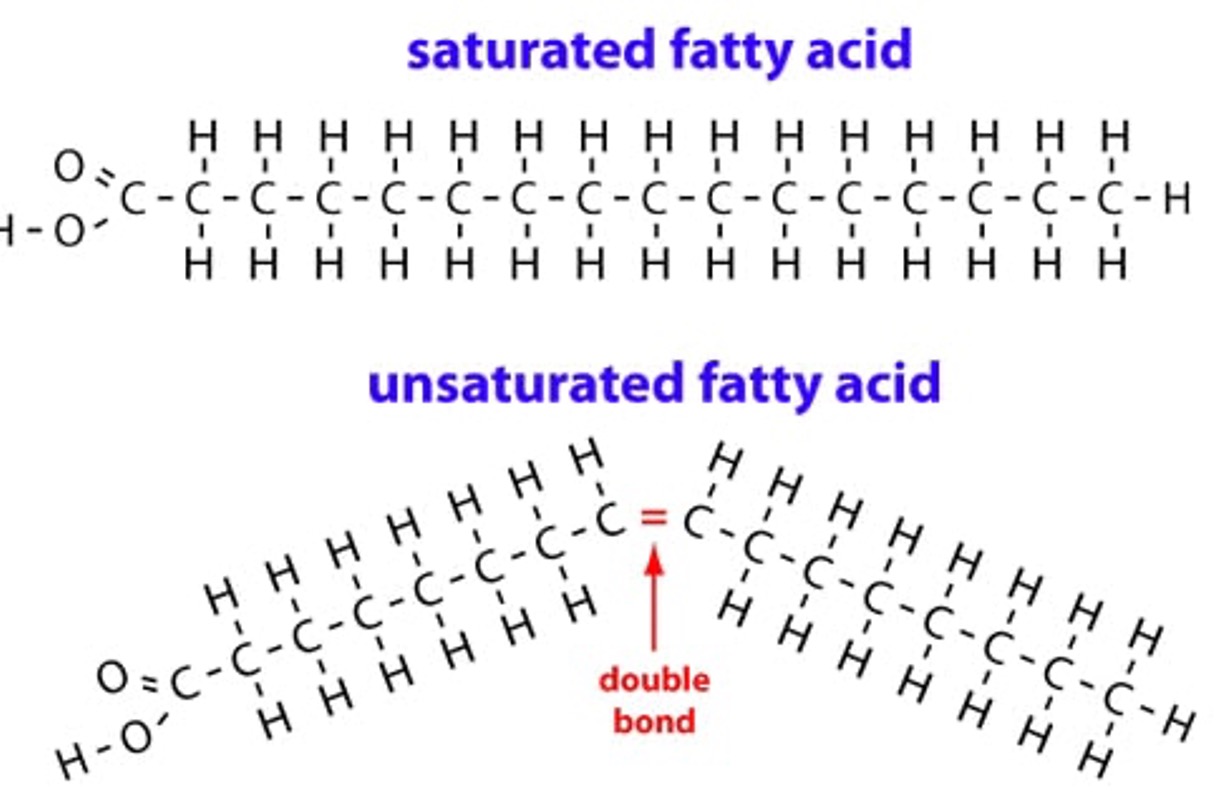 But what does this actually do?
But what does this actually do?
Bonding in fatty acids add changes to the properties. Lipids are stored either as fats (saturated) or oils (unsaturated)
Fats (Saturated)
–Come from endothermic organisms
Saturated fatty acids have straight chains that can be more tightly packed, making them more efficient for energy storage. However, this tight packaging also increasing the number of intermolecular forces between the fatty acid chains, resulting in a higher melting point
Oils (Unsaturated)
–Come from plants
–Unsaturated fatty acids have kinked chains that cause them to be more loosely packed (poly-unsaturated fatty acids have multiple kinks and are consequently even more dispersed)
–This means there are fewer intermolecular forces, and less energy is required to separate the fatty acids, resulting in a lower melting point
Triglycerides in adipose tissue for energy storage and thermal insulation
Triglycerides are used for long-term energy storage; whereas carbohydrates are short-term energy.
Triglycerides can store twice as much energy per gram than carbohydrates.
Triglycerides are more difficult to digest and are not easily transported because they are hydrophobic (this also means they don’t dissolve in body fluid).
Thermal insulation
Triglycerides have low thermal conductivity, meaning they are poor conductors of heat but very good thermal insulators.
Mammals living in cold or aquatic environments (such as the ringed seal) will possess thick layers of subcutaneous fat (blubber) to insulate their internal organs against cold exposure.
In humans, individuals with larger fat storages tend to cool less rapidly and have a form of insulation, however the increased retention of heat makes them more susceptible to heat stress.
Desert animals still need triglycerides for energy storage but tend to accumulate fat in areas that would reduce heat build-up; e.g. humps, tails.
Formation of phospholipid bilayers as a consequence of the hydrophobic and hydrophilic regions
Phospholipids are one of the key components of all cell membranes that are responsible for the formation of lipid bilayers. Phospholipids consist of a polar head (hydrophilic) composed of a glycerol and a phosphate molecule and two non-polar tails (hydrophobic) composed of fatty acid chains.Because phospholipids contain both hydrophilic (water-loving) and lipophilic (fat-loving) regions, they are classed as amphipathic.Phospholipids spontaneously arrange into a bilayer with the two hydrophobic tails being shielded from the surrounding polar fluids by the outward facing hydrophilic heads. The phospholipid bilayer is only held together by the weak hydrophobic associations between the non-polar tails, making the bilayer both fluid and flexible.
Ability of non-polar steroid to pass through the phospholipid bilayer
Steroid are a group of lipids with four rings of carbon atoms and 17 carbon atoms in total. The key differences is the addition and placement of different functional groups, e.g. –OH or –CH2
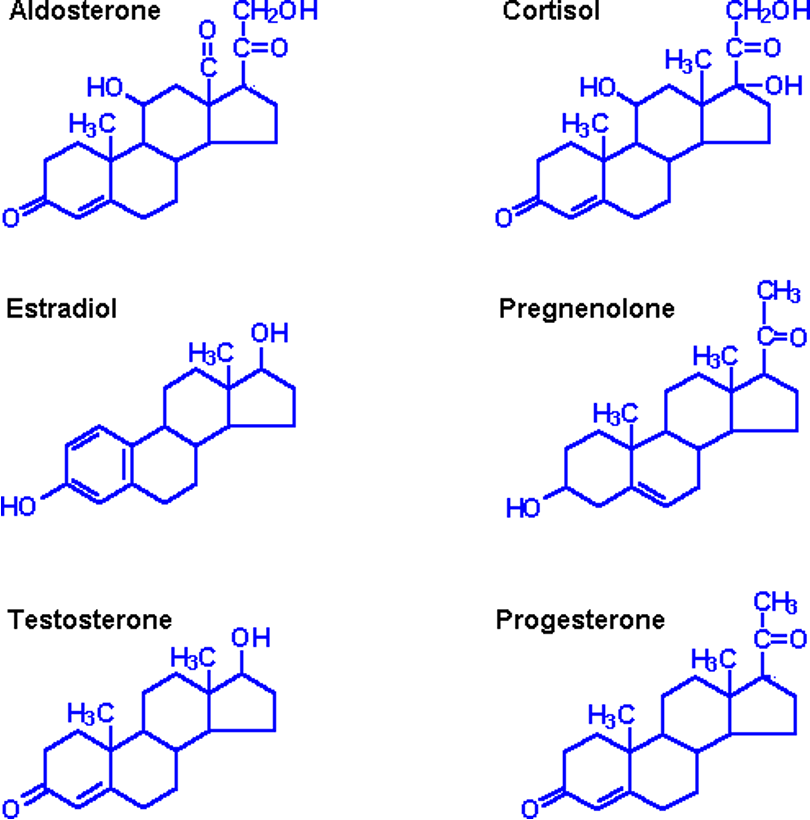
Steroids are hydrophobic and are made of hydrocarbons. Lipids are all non-polar and therefore don’t have an overall charge. Without a charge, water cannot form hydrogen bonds with non-polar molecules.Non-polar molecules are hydrophobic and will not dissolve in water. Lipids cannot attract water, they are insoluble. However, non-polar, hydrophobic molecules are able to diffuse directly through the hydrophobic core of the phospholipid bilayer. Once inside the cell, steroids bind to receptors inside the cell. Due to their hydrophobic nature, steroids cannot be dissolved in the bloodstream and must be transported bound to carrier proteins.