L3: Random Walks in Biological Systems
mmodels the path of a molecule as it undergoes successive, random steps.
Steps are independent and random in direction and magnitude.
underpin diffusion processes in cells.
One Dimensional Random Walk
number line: ← -\delta — 0 — \delta —>
t=0, x(0)=0
vx
1) once left/right
one step: \delta = ± vx\tau
\tau = duration of step
2) P→=P←=1/2
successive steps are independent (decrease collisions, decrease concentration, increase reaction vessel)
3) particles do not interact
one particle’s movement: xi(n) = xi(n-1) ± \delta
Average position of a molecule: mean squared displacement (MSD):
<x(n)> = 1/N \Sigma^N_i=1 (xi(n))
= 1/N \Sigma^N_i=1 (xi(n-1) ± \delta)
= 1/N \Sigma^N_i=1 (xi(n-1))
= <x(n-1)>
= <x(n+1)>
= 0
deltas will usually cancel out - average zero displacement
mean position does not change from step to step
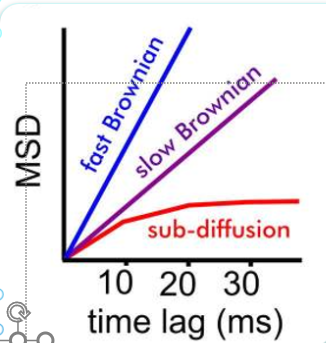
spread: root mean squared displacement (RMSD):
xi(n) = xi(n-1) ± \delta
xi2(n) = xi2(n-1) ± 2xi(n-1)\delta + \delta2
<xi2(n)> = 1/N \Sigma^N_i=1 (xi2(n-1) ± 2xi(n-1)\delta + \delta2)
= <xi2(n-1)> + \delta2
<xi2(0)> = 0 (by definition)
<xi2(1)> = <xi2(n-1)> + \delta2 = <xi2(0)> + \delta2 = \delta2
<xi2(2)> = <xi2(n-1)> + \delta2 = <xi2(1)> + \delta2 = \delta2 + \delta2 = 2\delta2
…
<xi2(n)> = n\delta2
t = n/\tau
n = t/\tau
<xi2(t)> = (t/\tau)\delta2
= (\delta2/\tau)t
\delta2/2\tau = D (diffusion constant)
<xi2(t)> = 2Dt
One-dimensional random walk equation
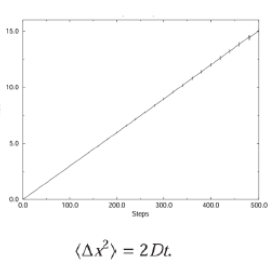
compare: x = vt
deterministic
random walk is always changing = unpredictable
describes one particle instead of spread
<xi2(t)>1/2 = (2Dt)1/2
lysozyme - D = 10-5 cm2/s
bacterial cell - 1 nm = 10-4 cm
<xi2(t)> = 2Dt
(10-4 cm)2 = 2(10-5 cm2/s)t
t = (10-4 cm)2/(2(10-5 cm2/s))
= 5x10-4 s
t = (1 cm)2/(2(10-5 cm2/s))
= 5x10-4 s
≈ 14 hr
Higher Dimensional Random walk
3D:
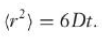
<r2> = 2nDt
where n is the number of dimensions
Diffusion
why do things diffuse? concentration gradients
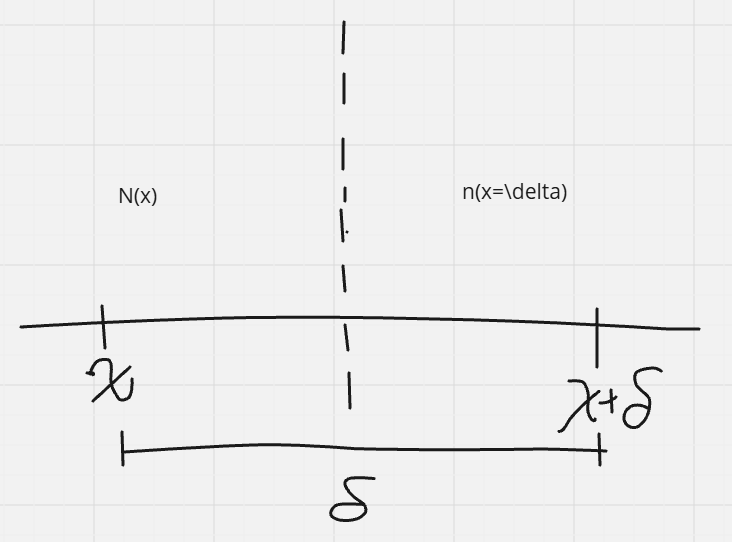
Jx(_L→R) = -1/2 [(N(x+\delta) - (N(x))/A\tau
A = area of wall
= -1/2 (\delta2/\tau\delta)[(N(x+\delta)/A\delta - (N(x)/A\delta)
= - D 1/\delta [C(x+\delta) - C(x)]
as \delta → 0
= -D ∂C/∂x Fick’s First Law
as \tau → 0
= 1/\tau [C(x+\delta) - C(x)]
= ∂C/∂t
as \delta → 0 and \tau → 0
∂C/∂t = D ∂2C/∂x2 Fick’s Second Law
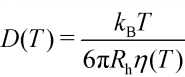
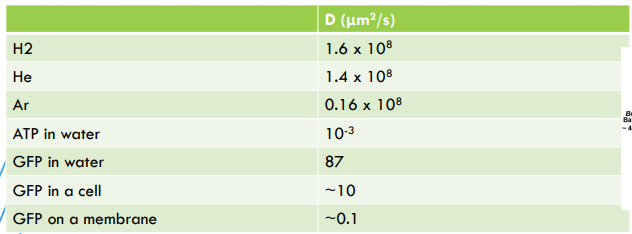
Key Takeaways
microscopic view - individual molecule stake random steps in a fluid
macroscopic view - collective behavior of many particles obey’s Fick’s Law
Brownian motion - concentration-driven diffusion
signalling molecules
drug delivery
neurotransmitter diffusion
fluorescence microscopy and protein diffusion