Electrons revolve around the nucleus in fixed paths called orbits/energy levels
An electron has a fixed/quantised amount of energy and it usually occupies the lowest energy level available
As long as the electrons remains in one energy level it neither loses or gains energy
When an electron absorbs energy it jumps from a lower energy level (E1) to a higher energy level (E2) and is now in an excited state
The electron is now unstable and the excess energy is released as light as the electron falls from (E2) to (E1)
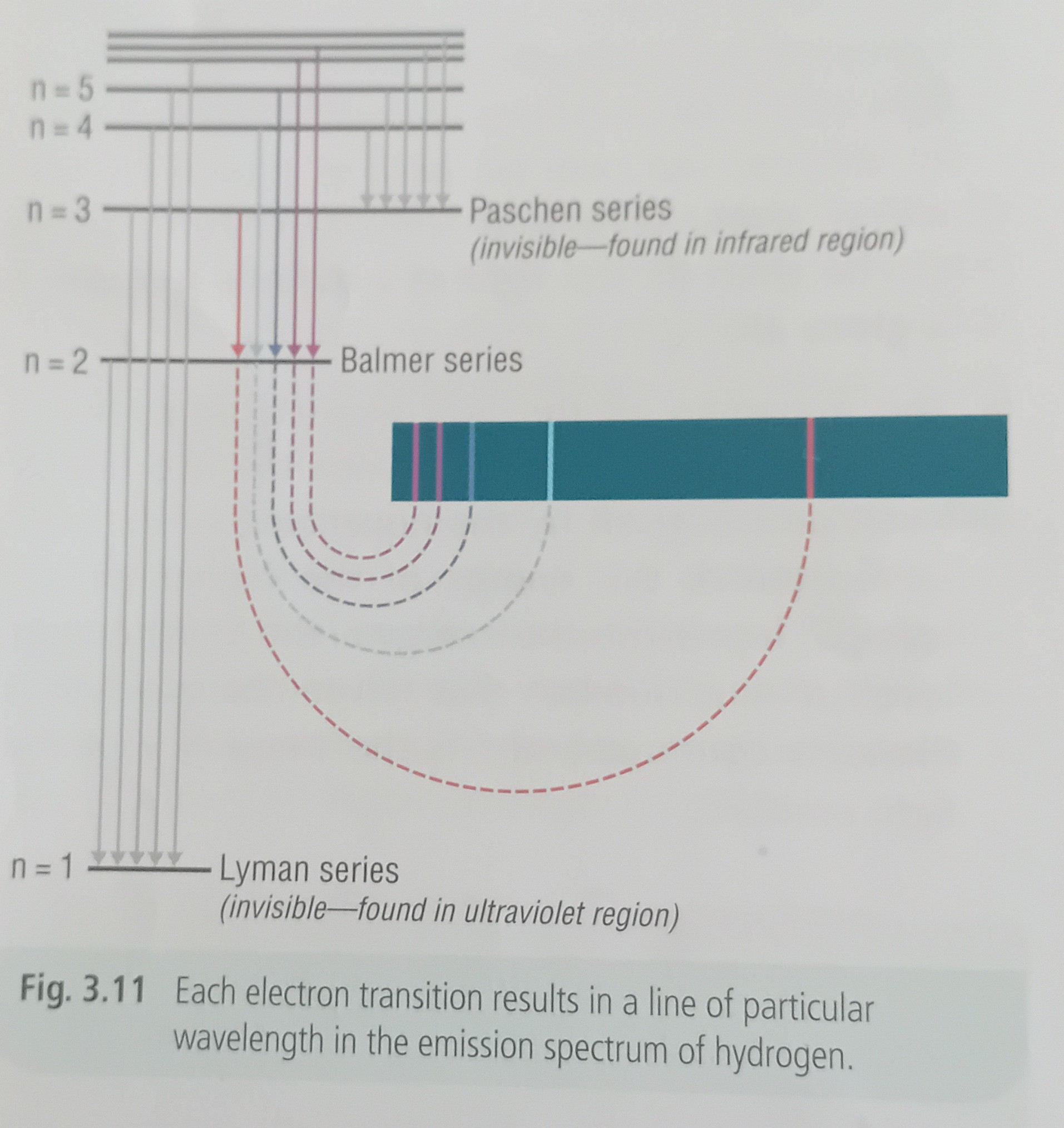
Light with a definite amount of energy lost → light of a definite frequency is given off
The frequency of light emitted depends on the energy difference between E1 and E2 → E2 - E1 = hf
Each definite amount of energy emitted gives rise to a particles line in the emission spectrum
Hence electrons must occupy definite energy level
Each element has a different number of electrons with a unique electronic configuration, there will be different numbers and types of electron transitions for each element which give rise to unique line emission spectra
 Note
Note Studied by 42 people
Studied by 42 people Note
Note Studied by 7 people
Studied by 7 people Note
Note Studied by 23 people
Studied by 23 people Note
Note Studied by 27 people
Studied by 27 people Note
Note Studied by 93 people
Studied by 93 people Note
Note Studied by 115 people
Studied by 115 people Knowt
Knowt