Thermal Processes
Conduction
- Conduction: The process by which heat or electricity is directly transmitted through the material of a substance
- Some substances (such as metals) are much better conductors than other substances.
Good conductors
The metallic structure is composed of a lattice of metal ions, and a sea of ‘free electrons’ that move throughout the structure.
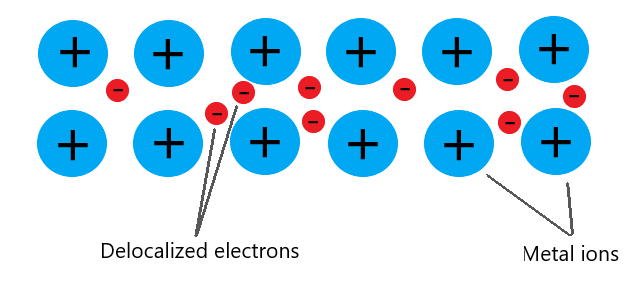
When one end of a metal is heated, the metallic ions gain energy and vibrate faster.
Ions are close together, so vibrations are passed onto neighboring ions which in turn, begin to vibrate quicker too.
Through the passage of kinetic energy in the form of vibrations, heat is transferred from the hot end to the cold end.
- The presence of free electrons is the main reason why metals are great conductors.
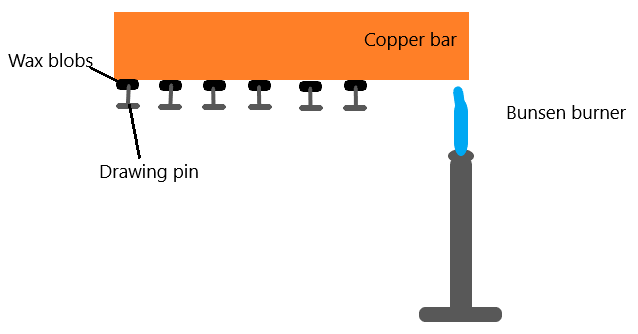
As the copper bar is heated at one end, the drawing pins will fall off one by one (from the one closet to the bunsen burner). This is because the metal conducts heat from the hot end to the cold end, and in doing so, melts the blobs of wax.
Poor conductors (insulators)
Liquids and non-metals are poor conductors of heat.
Insulators: Materials that are very poor conductors
- The absence of free electrons mean that molecules cannot easily pass on kinetic energy to their neighbors, so heat can only be transmitted through particle vibrations and collisions.
- The absence of free electrons is the reason why materials are poor conductors
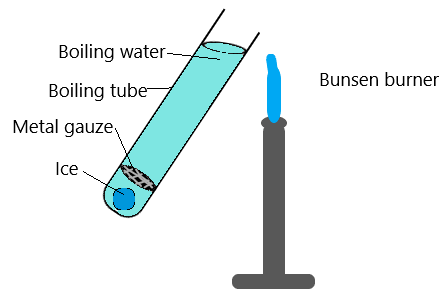
As the water at the top of the boiling tube is heated, it eventually boils. Despite that, the ice at the bottom of the tube does not melt.
This demonstrates that the heat is not reaching the bottom of the tube, and therefore that water is a poor conductor.
Convection
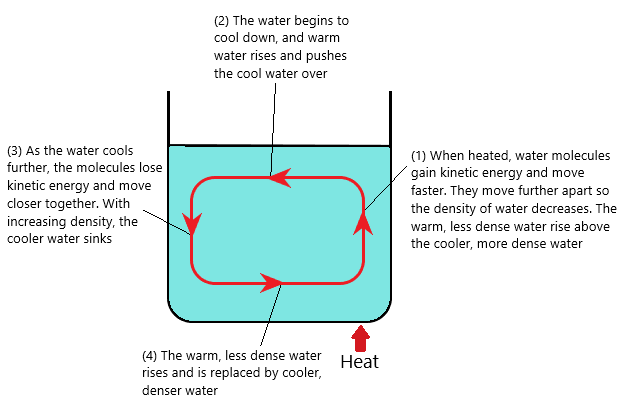
Convection: Heat transfer through liquids and gases (fluids)
When a liquid (or gas) is heated:
- The molecules push each other apart, making the liquid/gas expand
- This decreases the density of the fluif
- The hot liquid/gas rises, and the cooler (surrounding) liquid/gas moves in to take its place
- Eventually the hot liquid/gas cools, contracts and sinks back down again
^^The resulting motion is called a convection current^^
\n
Radiation
- Infra-red radiation is a part of the electromagnetic spectrum, and is emitted by any hot object.
- Infra-red radiation can travel across vacuum
- it does not need a medium for transmission.
- Infra-red radiation can be emitted, absorbed, or reflected.
^^Finding the best emitter of infra-red radiation^^
Set-up & procedure
- A metal cube with is painted with 4 different types of surfaces: matt black, shiny black, white and silver
- The cube is filled with boiling water
- A heat detector is placed at a constant distance away from the cube i.e. 50cm
- The cube is rotated so that each side faces the heat detector in turn, and the readings are noted
- Results:
- ^^Matt black (highest) → Shiny black → White →Silver (lowest)^^
^^Best absorber of infra-red radiation^^
Set-up & procedure
- A radiant heater is placed in the middle of two plates at equal distance away from the heater
- One plate is matt black and the other is silver
- A thermometer is placed on each plate and initial reads of the thermometers are recorded
- The heater is switched on and the temperatures of each of the plates are measured in equal intervals
- Results:
- The temperature of the matt black plate will increase quicker than the silver
- ^^Matt black is the best absorber and emitter of radiation^^