Unit 5; Bonding!
Topic One: Types of bonds and their properties
Ionic Bonds
- Made of a Metal & Non-metal
Hard but brittle because of Crystal Lattice Structure
- Dissolves in water easily
- Not conductive unless in liquid/aqueous state
- High melting and boiling points
ELECTRONS ARE TRANSFERED
Covalent (Molecular) Bonds
- Made of Non-metals
- Not conductive at all
-Soft
- Low melting and boiling points
ELECTRONS ARE SHARED
→ Network Solids are made of covalent bonds
Hard and high melting points
Metallic Bonds
- Made of Metals
- Good conductivity
→ due to mobile electrons
- Malleable and Ductile
ELECTRONS ARE SHARED
Topic 2: Naming Ionic Compounds
Review: Ionic bonds are between a Anion & Cation in which electrons are transferred from the Cation to the Anion.
A chemical formula tells you how much of each atom there is in a compound.
A subscript tells you how much of that specific atom there is.
- it’s a number that’s found on the bottom right
- you don’t show a subscript if there is only one of an atom.
Ionic compounds have a total charge of ZERO
Steps:
1. Find the oxidation state of a specific element on the periodic table.
→ for anions this is the first oxidation state; found on the very top
→ it is the same for cations that ONLY HAVE ONE OXIDATION STATE
2. Criss cross the charges.
3. Simplify to the nearest whole number and remove all the subscripts of one.
METALS ALWAYS GO FIRST IN THE FORMULA!!!!!!!!!!

Now here is how you get the oxidation number for the cations that have multiple oxidation states; mostly found in the transitional part of the periodic table (groups 3-12)
You can do this is in multiple ways;
#1; (probably the best method) SOS TABLE
First you make a table like this: (EX: CuCl2); find the oxidation charge of Cu
| Cu | Cl |
Subscript |
|
|
Oxidation # |
|
|
Solve | ||
Then; you plug in the subscripts for both elements; in this case it is 1 & 2 respectively
| Cu | Cl |
Subscript | 1 | 2 |
Oxidation # |
|
|
Solve | ||
Next, you plug in the oxidation # for both elements; since we don’t know Cu’s oxidation #, we can replace it with a variable, ill use X (Make sure to add the charge!)
| Cu | Cl |
Subscript | 1 | 2 |
Oxidation # | X | -1 |
Solve | ||
After that, you multiple the subscript by the oxidation #
So you would get 1x and -2 respectively.
You then make it into an equation; add them together to equal zero because REMEMBER!; ionic compounds have a total charge of ZERO!
so the final formula would be; X - 2 = 0
solve algebraically;
add two on both sides and
X = 2; so the oxidation # for Cu in this formula would be 2.
Your table should look like this.
| Cu | Cl |
Subscript | 1 | 2 |
Oxidation # | X | -1 |
Solve |
X = 2 | |
Heres another example for Mn02
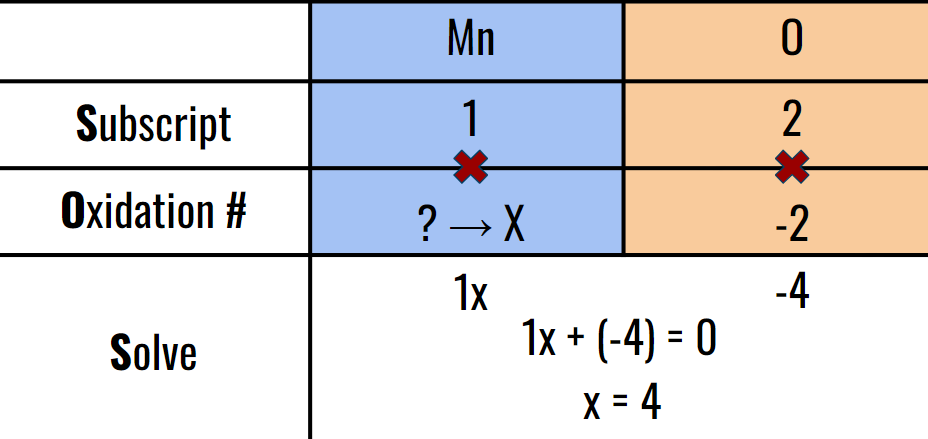
There is a quicker but less accurate and harder method though.
I don’t really have a name for it but here’s the steps
1. Identify if the formula is simplified.
(find anion’s oxidation # and see if it is a 1/-1)
2. if it is simplified then simplify it
Ex: Mn02
First; since oxygen’s oxidation # is NOT one, this is a simplified formula. So then what would cause a simplification to 2 that has a factor of 2? Four! because 4/2 = 2. So Mn would need to be a charge of 4 in order for the compound to simplify like that.
2b. if there is no subscript on the number it means that the anion and cation have the same oxidation # so if the formulas FeO, it would be Fe and O both have an oxi # of 2 since oxygen is -2 and they cancel out. (remember it’s always positive when in subscript form.)
3. if the compound is not simplified; you can do the reverse criss cross
Ex: CuCl2
Since chlorine’s oxidation # is -1, we can just swap the numbers so Cu would be 2 and Cl would be 1.
Ok, now to get to the actual naming part:
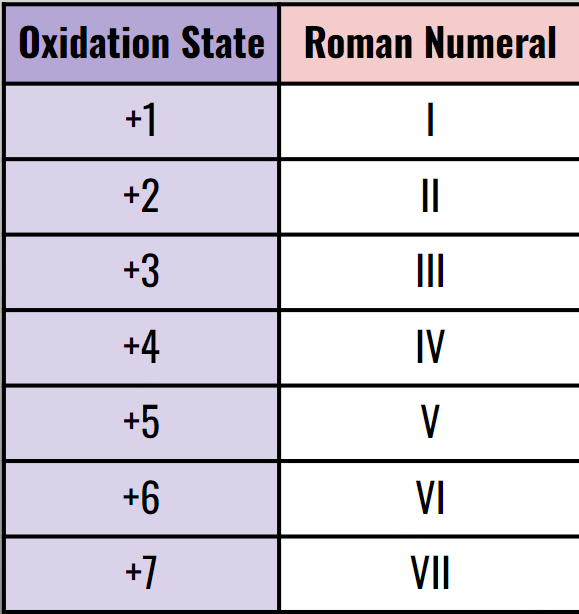
If the cation has multiple oxidation states; you need to write the # in roman numerals.
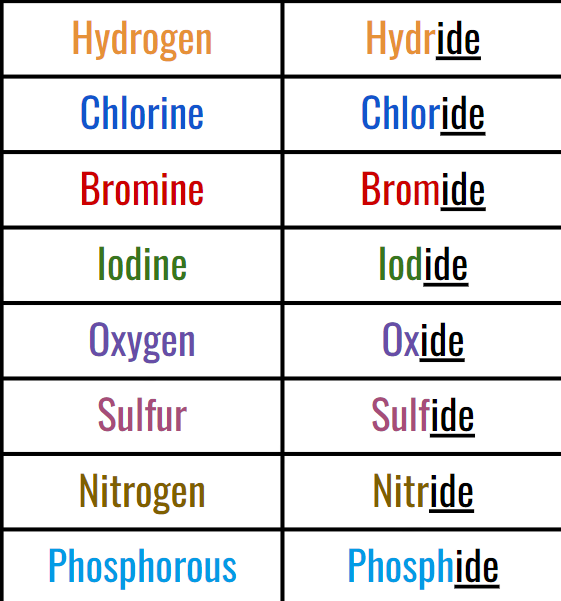
Then, for the anion, you add -ide to the name. (EX of common ones;)
So ex: MgCl2
so the name would be: Magnesium Chloride (since Mg doesn’t have multiple charges.)
Ex: FeO
So the name would be Iron (II) oxide
But if it’s Fe2O3
The name would be Iron (III) oxide
Topic Three: Naming Ionic Compounds with Polyatomic Ions
Polyatomic Ions are a group of charged atoms that stay together. You can find the ones that you need on Table E
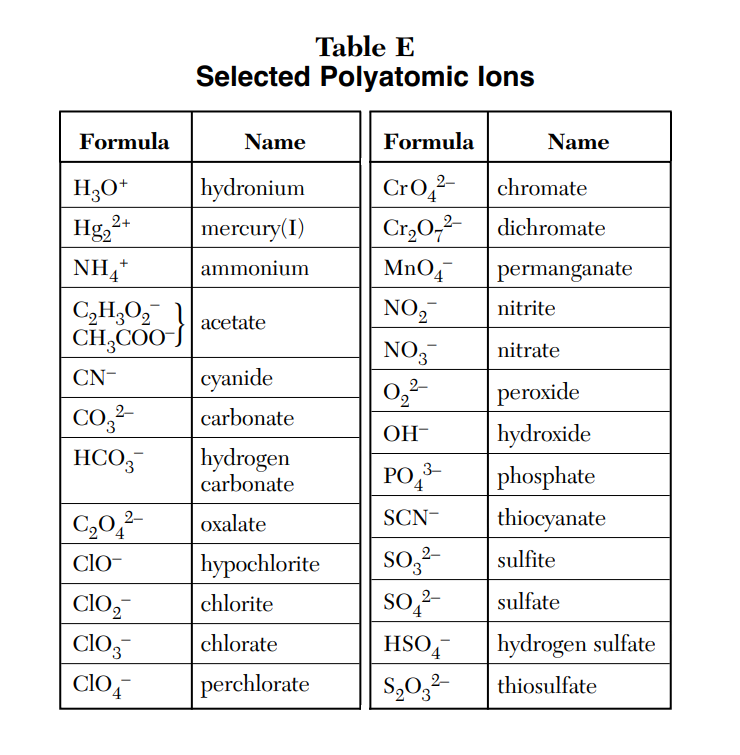
They replace the anion or cation in a Ionic Compound.
MAKE SURE NOT TO SEPERATE THE ATOMS IN A POLYATOMIC ION!
Chemical Formulas for Ionic Compounds w/ Polyatomic Ions
Find the Oxidation states of both the polyatomic and either cation or anion.
Criss Cross
Drop the ones & Simplify
(Just like the normal method that you use for naming regular Ionic Compounds)
Add parenthesis around the Polyatomic Ion if the subscript is greater than two.
Ex: Magnesium Nitrate
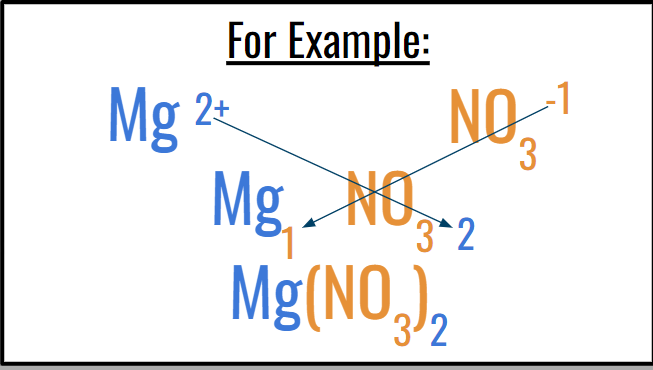
FAQ: “How do you know if it’s a polyatomic Ion?”
You literally js have to memorize them, you’ll get used to it the more you use them; some of them have specific suffixes though
Actual naming part:
Steps:
1. Name the Cation first
2. Put the Anion next with the -ide suffix
3. ONLY DO STEP TWO IF THE POLYATOMIC ION IS THE CATION! USE THE NAME ON TABLE E IF NOT!!!
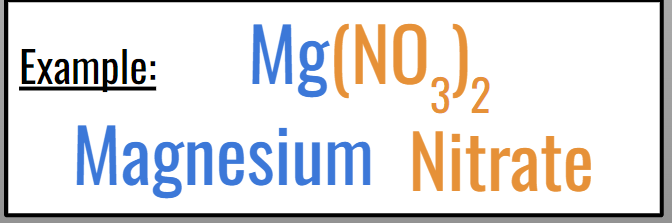
Ex: Mg(NO3)2
TOPIC 3: LEWIS DOT DIAGRAMS FOR IONIC COMPOUNDS
REVIEW: Lewis Dot Diagrams for atoms:
Put the element symbol in the middle, and the valence electrons are the dots, spread evenly.
Lewis Dot Diagrams for Ions
Put the element symbol in the middle,
CATIONS: only add the charge, no dots
ANIONS: add 8 dots, the charge and brackets
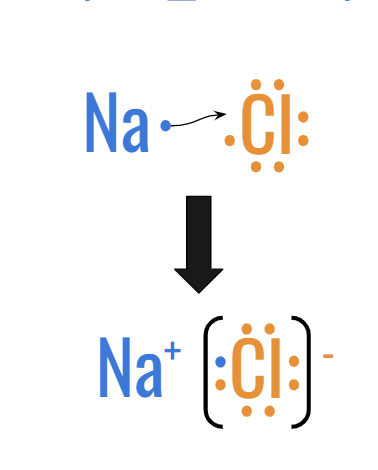
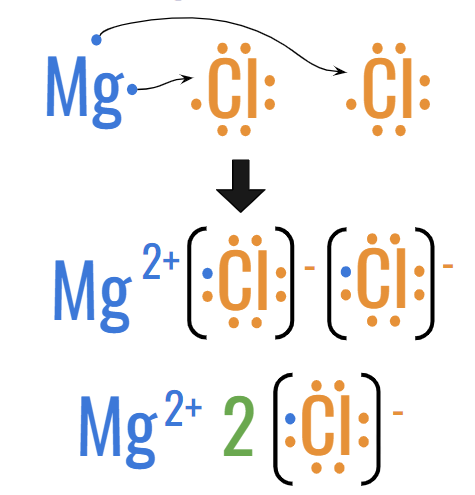
Lewis Dot Diagrams:
- Show the transfer of electrons from the Cation to Anion
Steps:
1. Draw the regular Lewis Dot diagrams for each element
2. Transfer the electrons from the metal to non-metal to create a Cation and Anion
3. Redraw
4. Add charges and brackets around the Anion
5. Add the positive charge for Cation
6. Add coefficient if there are multiple of a specific atom
Ex: NaCl Sodium Chloride (Table Salt)
Ex #2: MgCl2 (Magnesium Chloride)
Tips & Tricks:
The cation will always never have any dots and the Anion will always have 8 dots and brackets
So all you really need is the amount of the atom and the charge.
TOPIC 4: NAMING COVALENT COMPOUNDS
Steps of naming covalent compounds:
1. Name the non-metal’s (one that has lower atomic number goes first)
2. Use a prefix in the beginning:
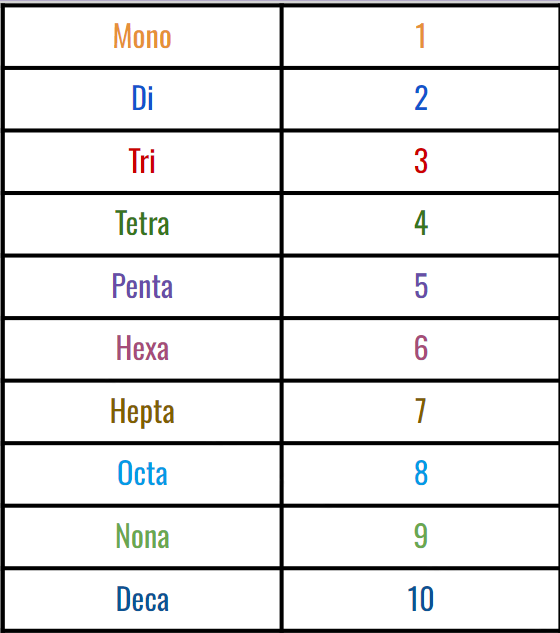
3. Change ending of second nonmetal to -ide (add suffix)
4. Do NOT USE mono- for the first non-metal
Ex:
1. SiO2
Silicon Monoxide
2. NO2
Nitrogen Dioxide
TOPIC 5: LEWIS DOT DIAGRAMS FOR COVALENT COMPOUNDS
Covalent Bonds
1. Single Covalent Bond (-)
2 shared electrons or 1 electron pair
2. Double Covalent Bonds (:::::)
4 shared electrons or 2 electron pairs
3. Triple Covalent Bonds
6 shared electrons or 3 electron pairs

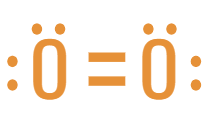
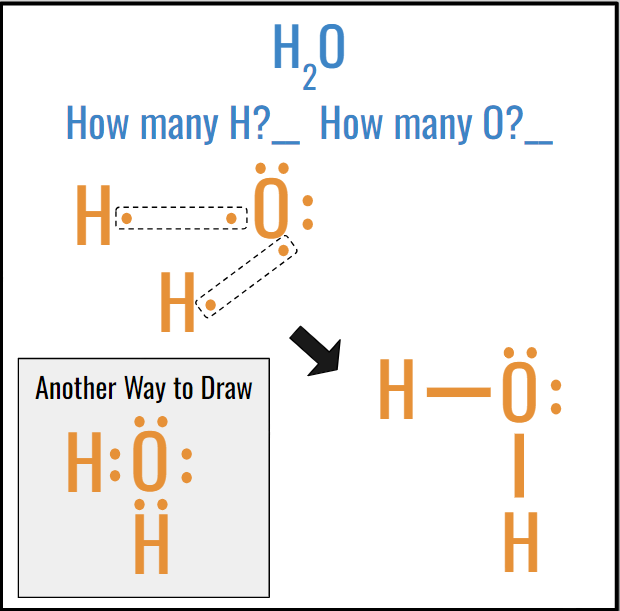
Actual Lewis Drawing Now:
1. Draw Lewis Dot Diagrams for each atom
2. Find the lonely electrons on each atom
3. Draw a circle around the lone electrons
4. Redraw
5. Draw a line to represent the shared electrons
6. MAKE SURE THE SHAPE IS CORRECT, THE CENTRAL ATOM’S LEWIS DOT DIAGRAM SHOULD BE FOLLOWING THE RULES OF A REGULAR, GO A SPECIFIC DIRECTION W/ VALENCE ELECTRONS
TOPIC 6: BOND POLARITY
Non-polar Covalent Bonds
→ Electrons are shared EQUALLY
- Consist of two of the same atoms
- Difference in electronegativity is ZERO (0)
Polar Covalent Bonds
→ Electrons are shared UNEQUALLY
- Consist of two atoms with different electronegativity values
The greater the electronegativity difference, the more polar the bond
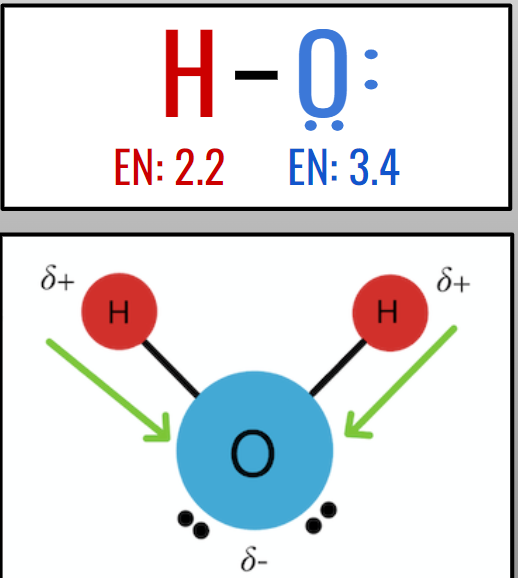
Charge Distribution
you can determine the charge of an atom in a molecule using electronegativity.
→ The more electronegative, the more partially negative (δ-)
→ The less electronegative, the more partially positive (δ+)
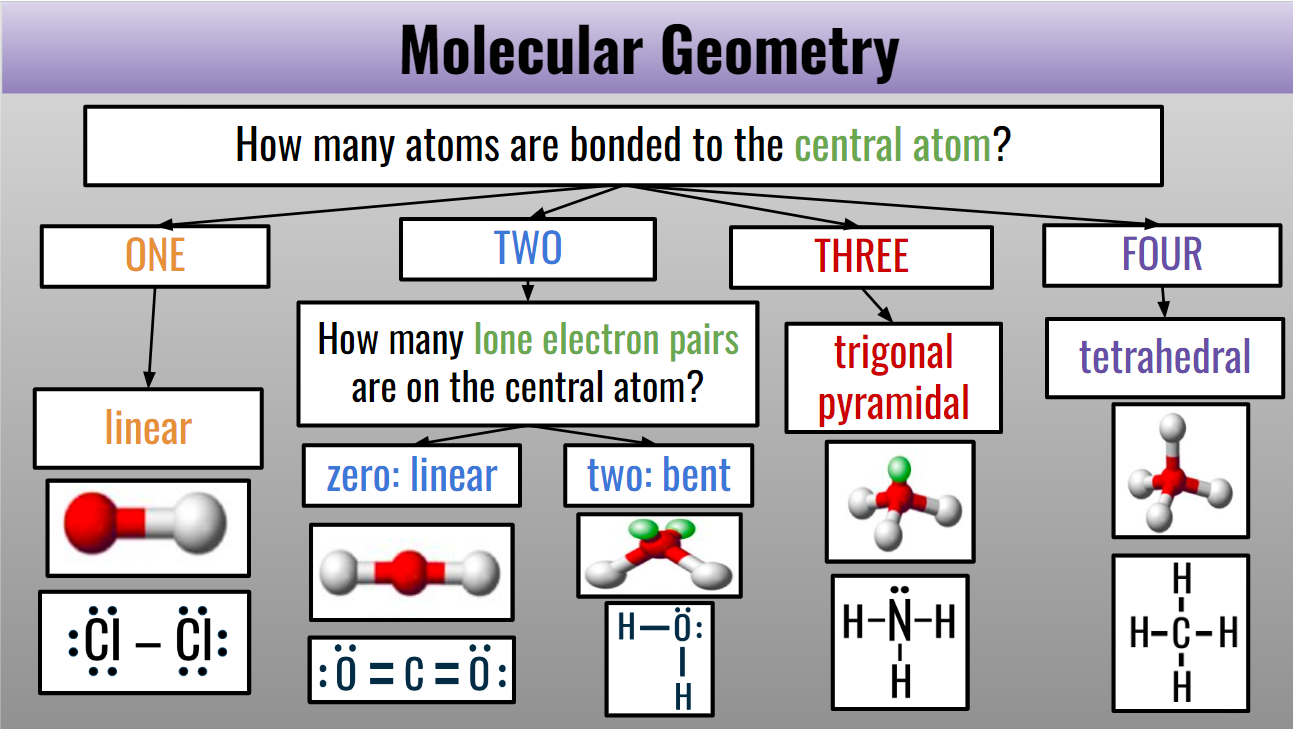
Molecular Shapes:
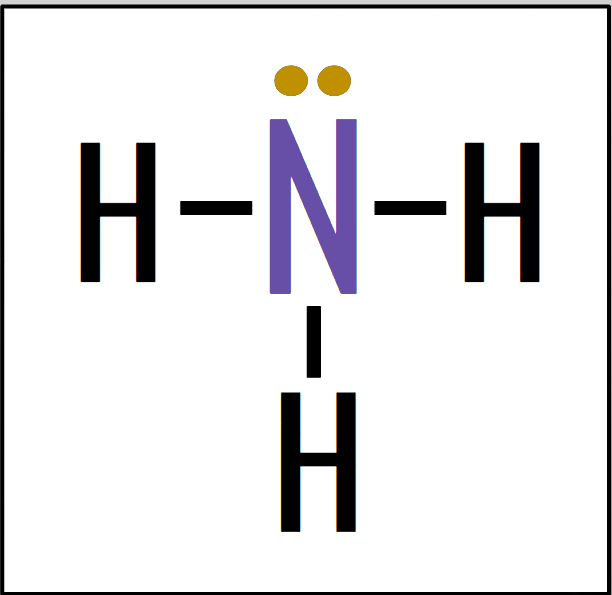
Central atom: found at the center of a molecule, usually is the unique atom (Purple)
Lone pairs: the electron pairs that aren’t bonded (Yellow)
Four Shapes:
Linear: Straight line (180 degrees)
Bent: literally the name, the molecule is bent
Trigonal Pyramidal: three atoms that are bonded to the central atom
Tetrahedral: four atoms that are bonded to the central atom
Molecular Polarity:
Equal Charge Distribution (Symmetrical) : Nonpolar
Unequal Charge Distribution (Asymmetrical): Polar
ACRONYM:
SNAP
S: Symmetrical
N: Nonpolar
A: Asymmetrical
P: Polar
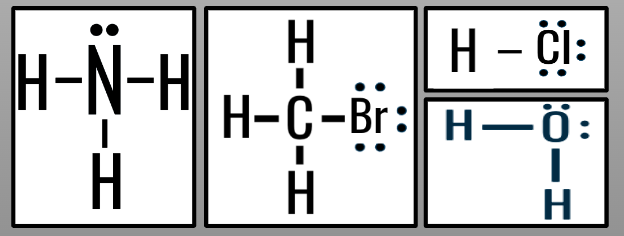
Asymmetrical Molecules
- asymmetrical charge distribution
→ atoms bonded to central atom are different
→ lone bonding pairs on central atom
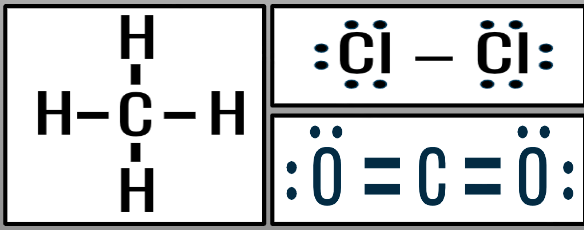
Symmetrical Molecules
- symmetrical charge distribution
→ atoms bonded to central atom are the same
→ no lone bonding pairs
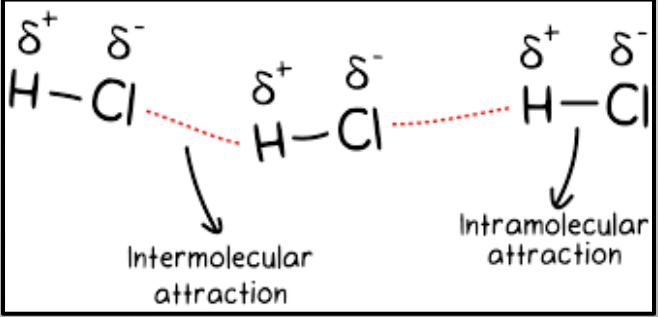
FINAL TOPIC: INTERMOLECULAR FORCES (IMF’s)
Intermolecular forces: attractive forces between two or more molecules
→ They are responsible for physical properties (melting point, boiling point, vapor pressure, and phase at room temp)
Solids
→ Strongest IMF’s because it’s closer together and harder to break apart (High Boiling Point)
Liquids
→ Medium IMF’s
Gases
→ Weakest IMF’s because the particles are further apart and easier to break apart (Low Boiling Point)
Types of IMF’s
→ WEAKEST
Van Der Waal forces (london dispersion)
- between two non-polar molecules
→ SECOND WEAKEST
Dipole-Dipole forces
- Between two polar molecules; partial negative attracts to partial positive
→ SECOND STRONGEST
Hydrogen bonding (IS STILL AN IMF DISPITE THE NAME)
- between hydrogen & Nitrogen, Oxygen, and Fluorine
You can remember this by: Hydrogen wants to have FON
→ STRONGEST
Ion-dipole forces
- Either between:
- Cation (+) & partial negative (δ-)
OR
- Anion (-) & partial positive (δ+)
they are the strongest because instead of using partial charges, the ions are fully negative and or positive.