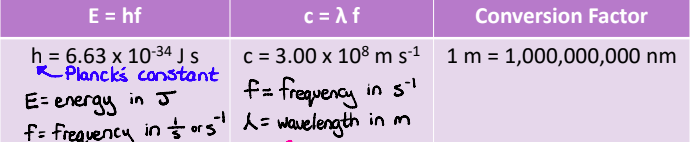1.3D The Limit of Convergence
The Limit of Convergence
Convergence: as energy levels in an atom increase, they get closer together until they converge.
The ground state is where an electron is with no addition of energy
The infinity level is the highest possible energy level that an electron can have and still be part of the H atom
If an electron exceeds n = ∞, electron is removed from attraction of nucleus and is no longer part of the atom
This forms a H+ ion
H(g) → H+ (g) + e-
 Calculating Ionization Energy from the Limit of Convergence
Calculating Ionization Energy from the Limit of Convergence
The limit of convergence is a wavelength in the emission spectrum of an atom
In an emission spectrum, it is where the lines stop (for a particular series)
The limit of convergence coincides with the amount of energy to get the electron to fly off
If we know the wavelength of the limit of convergence, we can calculate the energy that goes with that wavelength. This is the amount of energy required to remove the electron from the atom
First Ionization Energy: The energy required to remove one mole of most loosely held electron from one mole of gaseous atoms, to produce one mole of gaseous ions, each with a charge of +1