IUPAC System for Naming Alcohols
Rules for Naming Alcohols
- The parent name of the molecule is given based on the longest chain of carbons involving the alcohol group.
- The carbons in the chain are numbered to give the carbon attached to the alcohol group the lowest number.
- Change the suffix to -ol
- The alcohol must be numbered unless it is the only substituent in a cyclic molecule in which case it is at carbon 1 by default.
- There are two ways to name alcohols:
- you can put the number before the parent name as in 1-butanol
- you can put the number within the parent name as in butan-1-ol
Examples
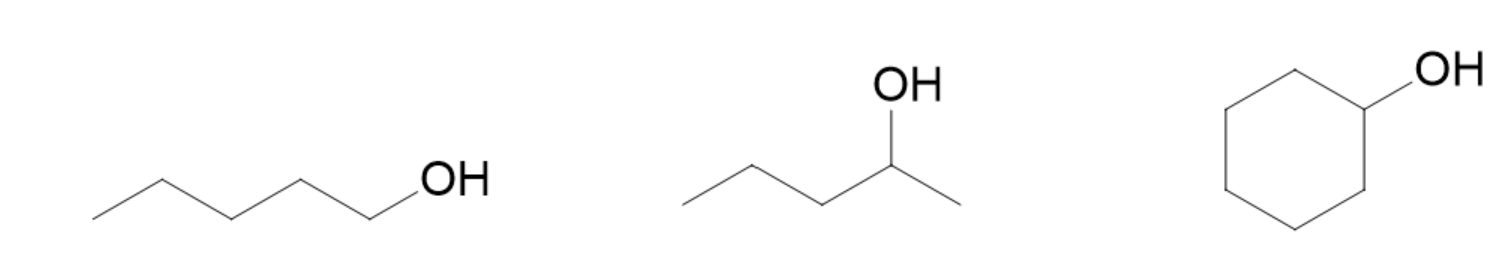
1-pentanol or pentan-1-ol
this is a 5-carbon chain with the alcohol group
to give the alcohol the lowest number, we number it from right to left to give the alcohol the number 1
- if we number from left to right the alcohol would be numbered 6
1-methyl-1-butanol or 1-methyl-butan-1-ol
the longest chain possible is 4 carbons counting from left to right and ending at the carbon where the alcohol group is attached
the chain should be numbered from left to right to give the alcohol the lowest number possible
- this gives the alcohol the number 1
the methyl group is also attached to carbon 1, so it is 1-methyl
cyclohexanol
- this is a cyclohexane with an alcohol group
- the alcohol group is the only substituent so it does not need to be numbered