3.1.7-8 Water & Ions
Unit 1: Biomolecules
3.1.7-8 Water and Ions
Key Notes
![]()
Name: ____________________
![]()
Hydrogen bond | Weak bonds between a slightly positively charged hydrogen atom in one molecule and a slightly negatively charged atom in another molecule. |
Dipolar | Having a positive and negative pole as a result of the uneven distribution of electrons within it. |
Metabolite | A substance involved in a metabolic reaction |
Solvent | A liquid that other substances dissolve in. |
Solute | A substance that has been dissolved in another substance. |
Specific heat capacity | The heat energy needed to raise the temperature of 1g of a substance by 1oC. |
Latent heat of vaporisation | The heat energy required to change water to water vapour. |
Cohesion | The attraction between molecules of the same type e.g. two water molecules. |
Adhesion | The attraction between molecules of different type e.g. a water molecule and the wall of the xylem. |
Surface tension | The measure of how hard it is to break the surface of a liquid. |
Ion | An atom with a positive or negative charge due to losing or gaining electrons. |
Water
Water is a major component of cells. Although very abundant, water is an extraordinary molecule with unusual properties which result from its polar nature.
Covalent and ionic bonding – a reminder
Covalent – Atoms share a pair of electrons in their outer shells, making a stable compound.
Ionic – ions with opposite charges attract one another. They are weaker than covalent bonds.
Water is a dipolar molecule:
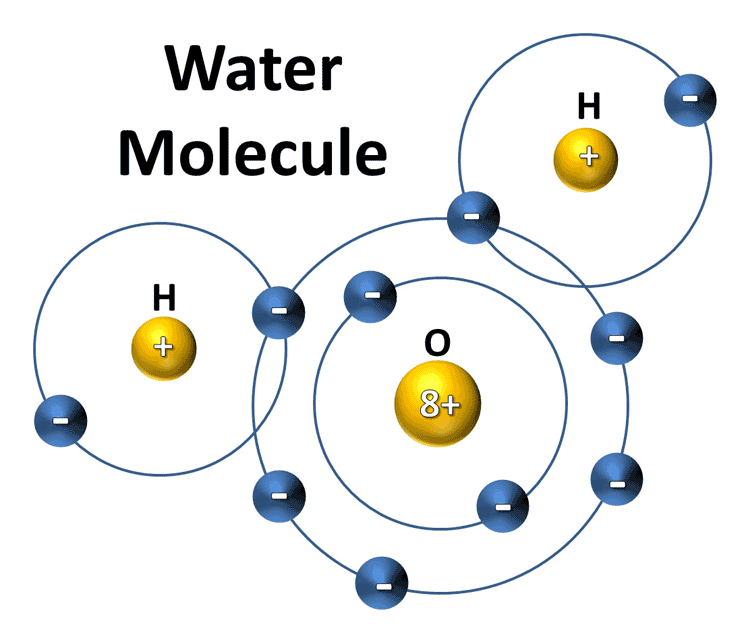 A water molecule consists of two hydrogen atoms and an oxygen atom; however, the electrons in the covalent bonding are not shared equally.
A water molecule consists of two hydrogen atoms and an oxygen atom; however, the electrons in the covalent bonding are not shared equally.The oxygen atom has a greater electronegativity, meaning that it has a greater pull on the electrons.
 Due to this each water molecule has slightly negative and slightly positive regions; the oxygen atom has a partial negative charge, while the hydrogen atoms are partially positively charged.
Due to this each water molecule has slightly negative and slightly positive regions; the oxygen atom has a partial negative charge, while the hydrogen atoms are partially positively charged.Therefore, water has both negative and positive poles; it is a dipolar molecule.
![]()
![]() Water and hydrogen bonding:
Water and hydrogen bonding:
The positive pole of one water molecule is attracted to the negative pole of another.
This attractive force between opposite charges is called a hydrogen bond.
A hydrogen bond is relatively weak.
However, in water there are huge numbers of hydrogen bonds, so they are a significant force and are responsible for many of the unique properties of water.
The importance of water to living organisms
Water is the main constituent of all organisms. The following properties make it incredibly useful.
Density:
Water is at its most dense at 40C.
When water freezes the hydrogen bonds between the molecules forms a rigid lattice, that holds the molecules further apart then in liquid water.
In ice, the molecules are spread out and form a crystal structure / lattice. So when water freezes it expands and so is less dense than liquid water and so ice floats.
Ice forms an insulating layer in many aquatic environments and acts as a barrier to the cold. The water below the ice does not freeze and remains as liquid water. This ensures aquatic organisms can still swim and survive.
![]() It is a metabolite:
It is a metabolite:
Water is a metabolite in many metabolic reactions – it is the medium in which enzyme-catalysed reactions take place and so is essential to maintain metabolism. Metabolic reactions occur faster in solution.
Water is used in hydrolysis reactions to break down complex molecules such as proteins to amino acids, and is formed in condensation reactions such as starch and protein synthesis.
Water is also one of the raw materials required in photosynthesis and is a product of respiration.
Universal solvent:
Water is an important solvent in which metabolic reactions occur.
An ion is a charged atom or molecule that has gained or lost electrons.
Ionic compounds are made from a positively charged atom and a negatively charged atom e.g. sodium and chlorine. There is very strong electrostatic attraction between the sodium and chloride ions in a salt crystal.
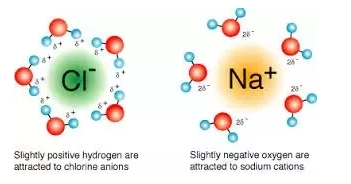 When they are dissolved in water, the positively charged sodium ion bonds with the slightly negatively charged oxygen of water and the negatively charged chloride ion bonds to the slightly positively charged hydrogen in the water molecules.
When they are dissolved in water, the positively charged sodium ion bonds with the slightly negatively charged oxygen of water and the negatively charged chloride ion bonds to the slightly positively charged hydrogen in the water molecules.The ions separate and the ions end up completely surrounded by water molecules keeping the particles in solution - this causes the sodium chloride to readily dissolve in water.
 Water is called the "universal solvent" because it dissolves more substances than any other liquid – this is due to its polar nature. This is beneficial for organisms as metabolic reactions occur faster in solution
Water is called the "universal solvent" because it dissolves more substances than any other liquid – this is due to its polar nature. This is beneficial for organisms as metabolic reactions occur faster in solutionMineral ions dissolved in water can be taken up by organisms e.g. nitrate ions can be taken into plants.
Note – you will learn about a wide range of ions as you progress through your A-level Biology course.
Water readily dissolves other substances such as:
respiratory gases i.e., oxygen and carbon dioxide.
excretory products such as ammonia and urea.
inorganic ions such as hydrogen, iron, sodium, chloride and phosphate ions
small hydrophilic molecules such as amino acids, monosaccharides and ATP.
![]() Cohesion, adhesion and surface tension:
Cohesion, adhesion and surface tension:
Water is ‘wet’ because it sticks to things. This is because its molecules can form hydrogen bonds with other polar substances.
Water can “stick” to itself or it can “stick” to other molecules and surfaces.
Cohesion | Adhesion | Surface tension |
The attraction between molecules of the same type. Water molecules are very cohesive because they are polar and have hydrogen bonds and so water molecules “stick” to one another. | The attraction of molecules of one kind for molecules of a different kind e.g. water “sticking” to the sides of a measuring cylinder. | The measure of how hard it is to break the surface of a liquid. At an interface between air and water, a water molecule on the surface forms hydrogen bonds with other molecules around and below it, but not with air molecules above it. The unequal distribution of bonds produces a force called surface tension; this causes the water surface to contract and form a surprisingly tough film or ‘skin’. Cohesive forces are responsible for surface tension. |
|
| |
These forces are important in the transport of water through xylem in plants, the drainage of tears from tear ducts in the corners of your eyes and some insects rely on surface tension to stay afloat on the surface of water.
![]() Water has a high specific heat capacity:
Water has a high specific heat capacity:
Water has thermal stability due to its high specific heat capacity.
Specific heat capacity is the amount of heat energy required to raise the temperature of a substance for a specific unit of mass e.g. the amount of heat energy needed to raise the temperature of 1kg of water by 10C.
Water molecules are bonded together by hydrogen bonding. These many hydrogen bonds require a lot of energy to separate them.
Therefore, water can gain or lose a lot of heat energy without changing temperature – it takes a lot of heat / energy to change the temperature of water due to these many hydrogen bonds.
This means that there is little variation in temperature of water either within organism’s bodies or in the water surrounding them.
The high specific heat capacity of water allows it to act as a buffer against sudden temperature changes.
The aquatic environment is therefore a temperature-stable one. As organisms are mostly water, it also buffers them against sudden temperature changes, especially in terrestrial environments.
The equation relating heat energy to specific heat capacity is given below (it does not need to be learned).
E = mcΔT
E is the heat energy transferred in joules, J
m is the mass of the substances in kg
c is the specific heat capacity in Jkg-1°C
ΔT is the temperature change in degrees Celsius, °C
Note - The specific heat capacity of pure water is 4190 Jkg-1oC.
If you wanted to find out how much energy in joules to the nearest whole number, must be transferred to raise the temperature of 3kg of pure water from 10°C to 15°C?
E = 3 x 4190 x 5
E = 62850 J
![]() Latent heat of vaporisation:
Latent heat of vaporisation:
When a substance changes from a liquid to a gas, it requires energy to do so.
The high number of hydrogen bonds between water molecules means that a lot of energy is required to evaporate 1g of water. This energy is called the latent heat of vaporisation.
This property of water explains why sweating is an effective means of cooling the body. When the body temperature rises above normal sweat is secreted. The water molecules in sweat gain energy. When a molecule gains enough energy, it can break free from the bonds that hold the liquid together and transform into water vapour. As the water evaporates, heat energy is removed from the sweat that remains on the body. This loss of energy cools the surface of the skin.
Inorganic ions
![]()
An ion is an atom that has an electric charge due to losing or gaining electrons.
These occur in solution in the cytoplasm and body fluids of organisms, some in high concentrations and others in very low concentrations.
Each type of ion has a specific role, depending on its properties.
![]()
Common ions we cover throughout the A-level course include:
hydrogen ions and their role in pH (more H+ ions = more acidic)
iron ions as a component of haemoglobin
sodium ions in the co-transport of glucose and amino acids
phosphate ions as components of DNA and of ATP.
.