Oxidative Phosphorylation P1
1. ATP: The Cell's Energy Currency
ATP → ADP + Pi releases -7.3 kcal/mol
Used as the immediate source of energy (not long-term storage).
Adult body contains ~100g ATP but recycles its entire body weight in ATP daily.
Hence, constant regeneration of ATP is essential.
2. Where the Energy Comes From
ATP is generated by coupling the transfer of high-energy electrons (from food) to phosphorylation of ADP.
One molecule of glucose produces ~30 ATP:
26 via oxidative phosphorylation
2 from glycolysis
2 from the TCA cycle
3. The Role of Oxygen
Oxygen is the terminal electron acceptor in the chain.
The challenge: safely and gradually transfer electrons to O₂ (a reactive species) without causing cellular damage.
4. The Electron Transport Chain (ETC)
Located on the inner mitochondrial membrane.
Electrons pass through a series of carriers with increasing redox potential.
Energy is released stepwise → used to pump protons into the intermembrane space.
5. Redox Potential and Free Energy
Electrons flow from low to high redox potential:
e.g., NADH (E°′ = –0.32 V) → O₂ (E°′ = +0.82 V)
ΔE = +1.14 V → ΔG = –220 kJ/mol
This energy is used to establish a proton gradient (not directly to make ATP).
6. Chemiosmotic Theory
Proposed by Peter Mitchell.
The proton motive force (PMF) generated by proton pumping drives ATP synthesis.
ATP synthase uses this PMF to convert ADP + Pi → ATP.
7. Structure of the Mitochondrion
Double membrane: outer (permeable), inner (impermeable, with cristae).
Cristae increase surface area for ETC.
Compartments:
Matrix: enzymes of TCA, fatty acid oxidation
Intermembrane space: site of proton accumulation
8. Electron Transport Chain Complexes
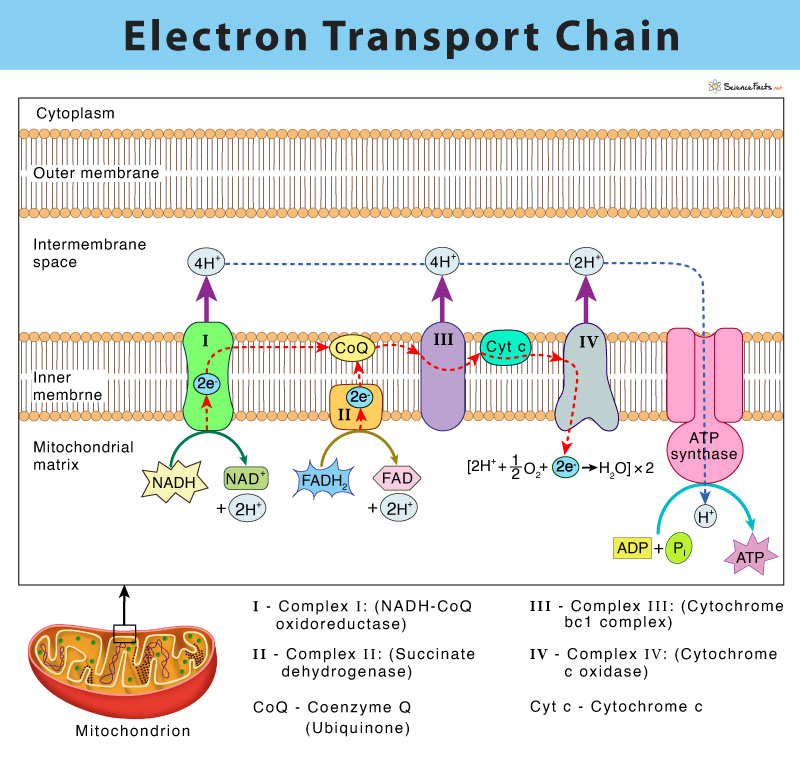
Complex I (NADH:Q oxidoreductase)
NADH → FMN → Fe-S clusters → Q → QH₂
Pumps 4 H⁺
Complex II (Succinate-Q reductase)
Succinate → FADH₂ → Fe-S clusters → Q → QH₂
No proton pumping
Q Pool
Mobile pool of ubiquinone (Q) / ubiquinol (QH₂)
Complex III (Q-cytochrome c oxidoreductase)
QH₂ donates e⁻ via Q cycle to cytochrome c
Pumps 4 H⁺
Cytochrome c
Carries electrons between Complex III → Complex IV
Contains heme group; mobile and conserved
Complex IV (Cytochrome c oxidase)
Accepts electrons → O₂ → H₂O
Uses heme a, a₃ and copper centers (CuA, CuB)
Pumps 2 H⁺ per 2 e⁻
9. Cofactors in the ETC
FMN: accepts 2 e⁻ from NADH
Fe-S clusters: 1 e⁻ at a time
Hemes (a, b, c): single e⁻ transfers
Copper centers: involved in O₂ reduction in Complex IV
Ubiquinone (CoQ): lipid-soluble, shuttles 1 or 2 e⁻
10. Mitochondrial Membrane Lipids
Cardiolipin: unique to inner mitochondrial membrane; stabilizes ETC complexes
PE and PC: structural support and membrane curvature
11. Endosymbiotic Origin of Mitochondria
All mitochondria evolved from an engulfed bacterial ancestor (e.g., R. prowazekii).
Retain bacterial-type genome and ribosomes.
✅ Summary of Key Steps (Biological Order)
Food → NADH/FADH₂ (TCA Cycle)
NADH/FADH₂ → ETC Complexes (I–IV)
Electron flow → proton gradient
Proton flow through ATP synthase → ATP
O₂ accepts electrons → H₂O formation