MCAT General Chemistry
Class 1 - 06/06/24:
Atoms - smallest unit of any element - has protons, neutrons, and electrons
p = +1, mass = 1amu
e = -1, mass = 0amu
n = 0, mass = 1amu
Atomic number = Z, the number of p*
Mass number = p*+n
The charge = p-e
Cations and anions are ions
C>0 = cation → +
C<0 = anion → -
C=0 = atom level
Isotopes: two atoms of the same element that differ in their number of neutrons - determined by mass number
Bohr model of the atom:
Electrons orbit at a fixed distance from the nucleus - the orbit decreases with distance from the nucleus - as we move away, the ends come closer and energy increases with distance from the nucleus
Electrons absorb only specific allowed E(due to fixed quantities of E)
Current orbit = ground state
Higher E orbit = excited state
Ephoton = Ef-Ei
e- in an excited state can come to a lower level to emit a photon - when dropped, the e- becomes relaxed
Hydrogen Absorption spectrum = dark bands on a bright background(absorb, so black line)
Hydrogen Emission spectrum = bright bands on a dark background(emit, so bright lines)
The Energy of a photon is related to its wavelength(λ lambda) and frequency(f)
E = hf = hc/λ → Wavelength and frequency are inversely related - when f is high, λ is low, E is high; when f is low, λ is high, E is low
E- exists in 3D orbitals and 4 quantum number describe their structures(orbitals are the areas around the nucleus where an e- is most likely to be found)
s, p, d, f → s being lowest in E and F in highest E
ex. boron= 1s2 2s2 2p1 → goes by block and row of the periodic table
3 basic rules for Electron filling:
Pauli principle: there can be no more than 2 e- in any given orbital(spin up and down)
e- cannot be the same, hence, different spins
Aufbau principle: E- occupies the lowest orbitals first and is filled in increasing E
exception is 3d and 4s → 4s are removed before 3d
Hund’s Rule: E- first occupy an orbital singly then pair up(no orbital left empty)
Anomalous electron configurations: when some elements prefer to be half-filled or filled by taking an e from 4s and putting it into 3d ex. Cr and Cu
Paramagnetic = at least one unpaired e-
Diamagnetic = all e- are paired ex. noble gases
Ground state e-configuration: the ground state is the lowest e configuration → correct amount of e as the element has
Excited state e-configuration: the element has jumped an orbital but it has not changed the amount of electrons as it originally had
A half-filled shell is more stable than one that isn’t; filled one is the most stable ex. noble gases
Valence shell configurations of elements determine the chemical reactivity of the elements → Elements in the same group show similar characteristics ex. noble gases as calm due to their octet shells, while halogens are reactive gases with 1 e-missing
Valence shell electrons experience electrostatic attraction due to the nucleus → shielding effect
force of electrostatic attraction is proportional to Zeff + C/r²
Atomic radius increases going down right to left
Ionization E increase going up from left to right
Electron affinity(negativity) increases going up from left to right
Electronegativity increases going up from left to right
FON=ClBrISC=H
Acidity increases going down left to right down
Class 2: 11/06/24:
Molecular Structure
Drawing Lewis structures:
count the number of valence e-
Arrange atoms with the least e-neg in the center(C is always in the middle and H is never in the middle) - Use FON=ClBrISC=H
Connect each outer atom to the center(each line is two electrons)
add dots of pairs to each electron till none are remaining
Complete missing octets
Assign formal charges
FC = valence e -(1/2 bonding e - sticks)-(lone pair e - dots)
Always check the number of e, octet rule obeyed, formal charges add up to total charge, the smallest set of FC, + charges on fewer e-neg atoms and - charges on more e-neg atoms
Sets of electrons also determine hybridization → count each bond and lone pair as one group
2 groups = sp linear ex. CO2
3 groups = sp² trigonal planar
4 groups = sp³ tetrahedral
The lone pairs on the molecule determine the molecular shape
AX4 = tetrahedral ex. CH4
AX3E = trigonal pyramidal ex. NH3
AX2E2 = bent ex. H2O
All have the same bond angles but different shapes
Chemical bonding: these bonds form when electrons are shared between two atoms as their orbitals overlap
More electrons shares make a stronger bond
A shorter distance between atoms makes a stronger bond
Stronger bonds have higher dissociation energies
Breaking bonds is always endothermic
Ionic or covalent bond types
Electronegativity determines the bond → if higher EN, then the bond is polar and e are not shared equally; if small EN, then the bond is shared equally and non-polar
Covalent bonds: formed between non-metal-non-metal → high EN - these compounds are insulators
Metallic bonds are formed between atoms with low EN metal-metal - these compounds are conductors and malleable
Coordinate covalent bonds are formed between atoms with lone pairs and e-deficient - ligands ex. hemoglobin
Ionic bonds are formed with particles of opposite charges anions-cations - these are insulators and brittle
Intermolecular Forces: when opposite charges attract each other - larger charges, stronger IMF and more tightly held together
Ion-dipole forces - between ions and polar molecules
Dipole-Dipole forces - between polar molecules and easier to cleave
Dipole-induced-dipole - between polar and non-polar - bigger e cloud and polarity from polar molecule but very easily cleaved
London Dispersion - temporary small dipoles formed by collisions - very weak
Hydrogen bonding - between very polar molecules with donors being (N-H, O-H, F-H) and acceptors being (O:, N:, F:)
Strongest bonds highest to lowest: Ionic>Covalent>Coordinate Covalent>Metallic
Strongest IMFs: Ion-dipoles>H-bond>Di-di>di-induced-di>LDF
Chemical Thermodynamics
Enthalpy(H) → The energy stored within chemical bonds or any attractive forces
the change in enthalpy of a reaction is the difference between energy stored in reactant vs products - delta H reaction can be high or low
Low H is exothermic(-) Forming bonds
High H is endothermic(+)Breaking bonds
Enthalpies of formation is the amount of energy associated with forming One mole of compound = sum of products-sum of reactants
Enthalpy is independent of its pathways - Hess’s law requires a combination of two or more reactions to find the enthalpy for the overall reaction
Entropy(S) = potential randomness - more particles, changing phases, increase temperature → all increases entropy
Gibbs free energy: energy available to do work
G = H - TS
Spontaneous process is exergonic → G is negative
non-spontaneous is endergonic → G is positive
Class 3 - 27/06/24:
Phases transitions → solid, liquid, gas, ideal gas
The IMFs decrease as the substance becomes more ‘liquidy’
Heat is absorbed going to gas, and released going to solid
Triple Point: the point where all 3 phases coexist
critical point: where the difference between liquid and gas is no longer distinct
Calorimetry: the science of measuring the changes to determine heat transfer - using a calorimeter
Heat of transition: the amount of E to complete a transition
heat of fusion: the amount of heat to be absorbed to change from solid to liquid
Heat of vapourization: teh amount of heat to be absorbed to change from liquid to gas
ΔH = molar enthalpy of change(kJ/mol)
n = number of moles of substance
q = mcΔT → adding heat can also raise the temp of a substance by increasing the kinetic E
Density: a measure of how condensed a substance is
p = m/v
IMF is inversely proportional to p
Boiling point: the temperature at which the condensation/vapourization phase transition happens
Melting and freezing point: the temperature at which fusion and crystallization occurs
Solution: a homogenous mixture of two or more substances → solvent is usually water
Electrolyte: a solute that is dissolved
van’T Hoff Factor: the number of articles produced in a solution per mole of a substance
electrolytes dissolve in water
polar non-electrolytes dissolve in water
non-polar non-electrolytes do not dissolve in water
Like-dissolves-like
Solubility: the amount of a substance that can dissolve in a specific solvent at a specific temperature
Unsaturated - too cold → still can add more solute
Saturated - just right → solvent=solute
Supersaturated → too hot → too much solute, produces a precipitate
Electrolytes in WATER:
Ideal gas: has ZERO IMFs - particle size is negligible volume compared to container size
Has Ek proportional to temp
ideality favoured with high temp and low pressure
Avogadro’s law: the volume of an ideal gas is proportional to the number of particles in the container at the given time, regardless of the identity of the gas
STP = 0°C and 1 atm
Two variable Gas Law’s
Boyle’s Law: P is inversely related to V → p1v1 = p2v2
Charles Law: t is directly proportional to V → v1/t1 = v2/t2
Gay-Lussacs Law: P is directly proportional to T → p1/t1 = p2/t2
Pressure: 1atm = 1000mL = 1000 cm³ = 0.001 m³
Temperature must be in the absolute temperature scale = Kelvin → °C + 273 = K
Combined Gas Law → Boyle’s and Charle’s
p1v1/t = p2v2/t
Ideal Gas Law: PV = nRT
R = gas constant(0.08 Lxatm/Kxmol)
Ideal pressure will always be greater than real pressure(since real gas do experience IMFs)
Ideal volume will have more free space in a container than a real gas
Dalton’s Law: states that the total pressure of a mixture of gases is equal to the sum of their partial pressures
Graham’s Law of Diffusion/Effusion: the rate of diffuse/effusion of a gas is inversely related to the square root of its molar mass
Rate gas 1/rate gas 2 = √(molar mass gas2/molar mass gas1)
Heavy particles move slowly and light particles move quickly
At 1 mole He gas, takes up 22.4L of volume at ideal temperatures
P vs. T = direct relationship → higher pressure, higher temperature
P vs. V = indirect relation → higher pressure, lower volume
V vs. T = direct relation → higher volume, higher temperature
P vs. n = direct relation → higher pressure, moles increase
V vs. n = direct relation → as volume increases, moles increase
Class 4 - Kinetics and Equilibrium
Reaction Coordinate graph
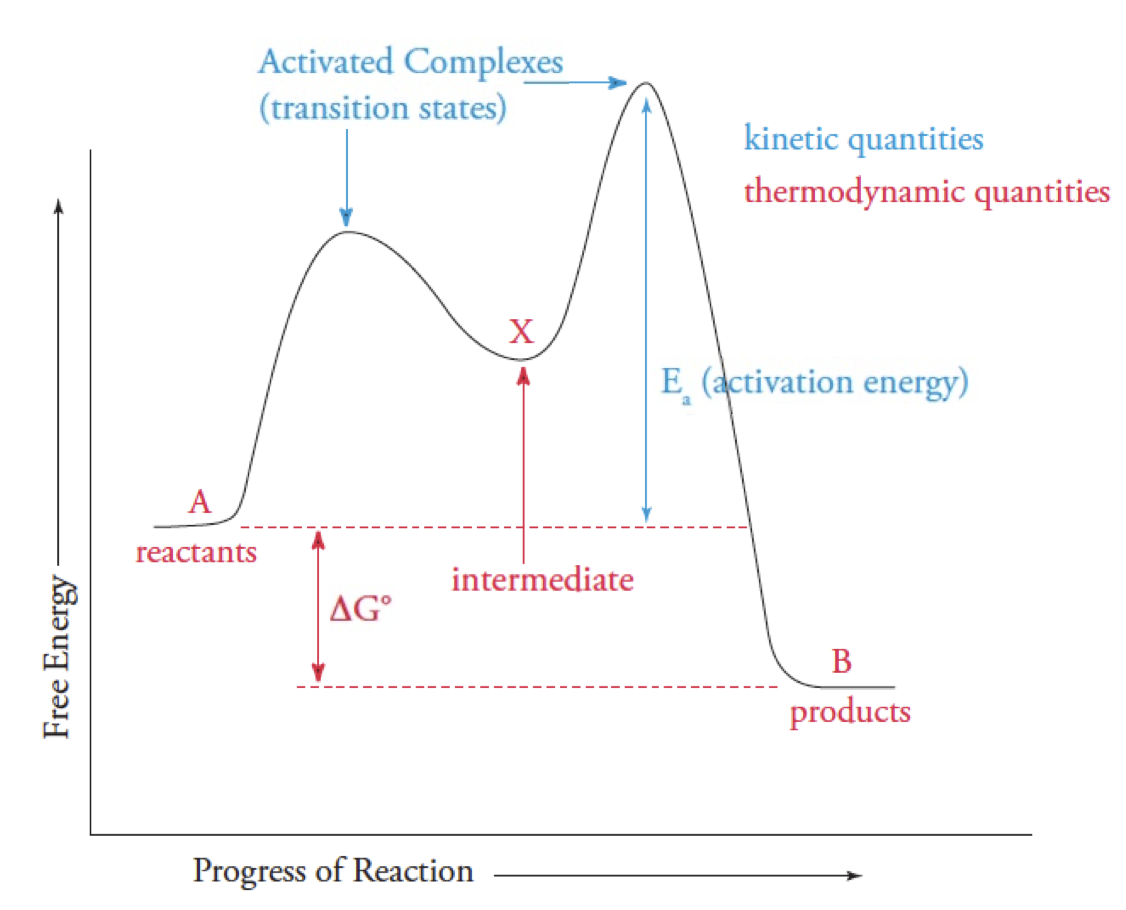
Ea is the difference between the reactant E and the highest E transition
Highest Peak = slowest step(rate-limiting step)
Reaction rate = change in concentration of a reactant or product over a change in time
rate = -1/r x [R]/t = + 1/p x [P]/t → reactants will be (-) and products positive since we gain them
The rate of the reaction will always have a positive rate in the forward direction
rR → pP
Anything with the same coefficients will have the same rate of change
Rate of reaction = to the compound in the stoichiometric equation with a 1 in front of it
Collision Theory: reactants must collide at a faster rate
Increase temp
increase pressure, lower volume
increase concentration of reactants
adjusting molecules to a proper orientation by the addition of a catalyst → quicker reaction
cause activation E to change → lower it by adding a catalyst or increasing temperature
Rate constant = the more successful the reaction, the higher the K value
K is inversely proportional to the Ea → Ea higher, lower K
K is directly proportional to the temperature
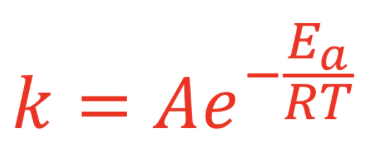
Reaction Mechanism:
Catalysts
Intermediate
Transition states
Rate Laws: give rate in terms of initial [C] and rate constant for the process
Rate = k[R]^r rR → pP
Only for elementary steps and not the overall reaction and solids and solvents not included
Write out rate law, make sure powers of 10 are the same, and make sure the order of two different trials is the same
0th - no collision - different [C] but same rate overall
1st - one collision - double [C], doubles rate
2nd - anything else that doesn’t match
rate constant can be found from a single trial
Dynamic Equilibrium = forward and reverse rates are equal - [C] will be consistent at equilibrium
K = [P]^p/[R]^r → K can only be calculated at equilibrium
Any K value will always be at equilibrium → K is unitless
K >1 → More products
K < 1 → More reactant
K = 1 → Both P and R are the same
Kc = [C]
Kp = pressure
Kb = dissociations
Ka = acids
Kb = base
Kf = formation
Ksp = solubility
Reaction Quotients → described the distance from Equilibrium → ratio of initial product/reactant [C]
Q > K → more products, the reaction goes in the reverse direction
Q = K → at equilibrium
Q < K → more reactants, the reaction goes in the forward direction
Delta G = G° + RT lnQ
Q>K → reverse → delta G is + → non-spontaneous in forward, but spontaneous in reverse
Q<K → forward → delta G is - → spontaneous in forward direction
Delta G = 0 → Q=K at equilibrium
delta G° = -RT lnK
K»1 → more products, delta G° becomes negative
K«1 → more reactants, delta G° becomes positive
K = 1 → delta G° is 0
Le Châtelier’s Principle
a system in equilibrium, will shift to decrease stress when stressed
Solids and liquids don’t affect equilibrium
Increase V, less pressure → shift to more moles of gas side
Decrease V, increase pressure → shift to less gas side
Treat Temperature like a product/reactant
If Endothermic, heat is → used up → reactant
+ delta H
If Exothermic, heat is → produced → product
- delta H
Temp change Q and K → only way to change K value(applies to all K values)
Multiple Equilibria → Some things can change other reactions(chain reaction)
At equilibrium, forward = reverse
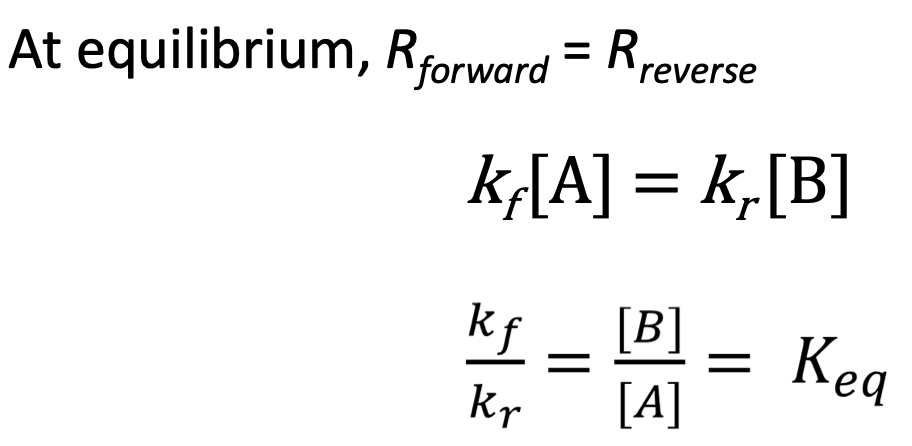
Formation Equilibrium → equilibrium of a coordination complex
Reversing equilibrium
Combining equilibrium means multiplying the K
Solubility Equilibrium: dissolving salts into water → cationic and anionic parts
Ksp(solubility) → use of ions only that are aqueous
Molar solubility = mol solute/L solution
More molar solubility, more solubility of the salt
ICE Tables
Qsp = [Ion A][Ion B]…
Qsp > Ksp → supersaturated → can form precipitate
Qsp = Ksp → saturated = equilibrium
Qsp < Ksp → unsaturated
Common Ion effect: salt’s solubility decreases if a common ion is added to the solution
If add an acid, acid/base reaction could happen → solubility will increase if common ion removed
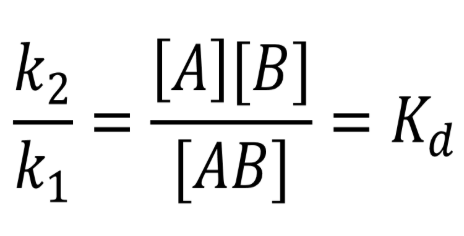
Kd = dissociation
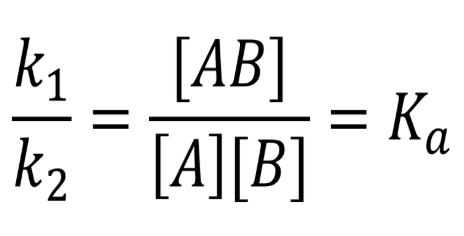
Kaff → affinity
putting it together, reaction reversed → inverse of the dissociation constant
Kaff = 1/Kd
Kinetics = rate, mechanism, catalyst, Ea
Thermodynamics = stability, equilibrium, spontaneity, enthalpy/entropy, free E
Class 5 - Acids and Bases