SCIENCE TRIMESTER 1
DESCRIBING AND CLASSIFYING MATTER
Matter is anything that has mass and takes up space.
Chemistry is the study of matter and how it changes.
One of the first step in classifying matter is to determine whether something is a substance- a single kind of matter that always has a specific makeup, or composition.
PHYSICAL AND CHEMICAL PROPERTIES
A physical property is a characteristic that can be observed without changing the matter into another type of matter
EX. When boiling a gold bar, the substance doesn’t change. When it melts it is the same gold bar, just in a different state. Since the substance didn’t change, it is a physical property.
A chemical property is a characteristic that describes something’s ability to become something else.
EX. If one were to inject CO2 gas into liquid water, there would be a reaction producing carbonic acid. The ability to react is a chemical property of water. Likewise, the ability of carbon dioxide to combine with water and become carbonic acid is a chemical property.
COMPONENTS OF MATTER
The particle theory of matter explains that all matter is made up of tiny particles called atoms.
An atom is the basic unit from which all matter is made. Different substances are made up of different types of atoms. However, all atoms have the same basic structure
An atom has a positively charged center, or nucleus, containing smaller particles. Negatively charged particles around the nucleus and form a cloud of negative charge.
A chemical bond is a force of attraction between to or more atoms. These form when atoms combine with other atoms. Sometimes they even form extended structures. One of these are molecules
A molecule is a group of two or more atoms held together by chemical bonds.
COMPOUNDS
A compound is a substance made of two or more elements that are chemically combined in a set ratio.
A compound is represented by a chemical formula, which shows the elements in the compound and ratio of atoms.
EX. CO2.
The ratio here would be 1:2 since there is ONE carbon atom, and 2 oxygen atoms.
TYPES OF MIXTURES
A mixture is made up of two or more substances that are together in the same place, but their atoms are not chemically bonded
A homogenous mixture when it is difficult or impossible to see the different parts of the mixture.
A heterogenous is when substances are mixed together, but they are not chemically bonded to each other.
If one were to separate a mixture, they would have to divide its parts according to their properties. The methods one could use include distillation, evaporation, filtration, or magnetic attraction.
Magnetic attraction involves holding a magnet near a mixture to pull out anything attracted to the magnet, such as metal
Evaporation to separate dissolved or suspended substances from water.
Filtration when a substance is passed through some kind of filter that allows fine particles, such as molecules of water, to pass through while filtering out larger particles.
Distillation involves separating liquids by boiling them. The liquid with the lower boiling point will vaporize first, leaving the other liquid behind.
MEASURING MATTER
Mass is the amount of matter in an object. An object’s mass does not change even if the force of gravity upon the object changes.
Volume is the amount of space that matter occupies.All forms of matter-solids, liquids, and gases— have volume.
If one wants to measure matter, one needs to measure both mass and volume.
For this, first we need to consider weight.
Weight is a measure of the force of gravity on an object. The force of gravity depends on where its being measured.
REM: MASS DOES NOT CHANGE WITH LOCATION EVEN IF THE FORCE OF GRAVITY CHANGES!!
DETERMINING DENSITY
Density is a measure of the mass of a material in a given volume.
Density can be expressed as the number of grams in one cubic cm. (g/cm3)
To determine the density of a sample of matter by dividing its mass by its volume.
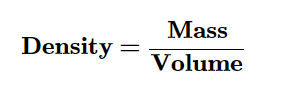 Temperature affects density.
Temperature affects density.
USING DENSITY
Since density does not change with the shape of an object, density can be used to identify substances.
CHANGES IN MATTER
PHYSICAL AND CHEMICAL CHANGES IN MATTER
A physical change alters the form of appearance of matter, but does not turn any substance in the matter into a different substance.
A chemical change produces one or more new substances.
CONSERVATION OF MASS
In the 1770s, a man called Antoine Lavoiser measured mass both before and after a chemical change. His data showed that no mass was lost or gained during the change.
This is referred to the Law of Conservation of matter.
The law of conservation of matter refers to the fact that matter is not created nor destroyed in any chemical or physical change.
ENERGY AND MATTER ARE RELATED
Energy is the ability to do work of cause change.
Like matter, energy is conserved in a chemical change. Energy is never created nor destroyed, it is only transformed.
Temperature is related to the motion and energy of the particles of matter.
THERMAL ENERGY AND ENERGY ARE RELATED
The total energy of the motion of all of the particles in an object is known as thermal energy.
When matter changes, thermal energy is usually released or absorbed.
Endothermic change occurs when energy is absorbed.
Exothermic change occurs when energy is released.
STATES OF MATTER
Matter exists in different forms, depending on a variety of factors, such as temperature.
DESCRIBING SOLIDS
A solid has a definite shape and definite volume.
The particles of solids are very packed together. So since they cannot move around, they vibrate slightly in place.
This is the particle arrangement of solids.
Types of Solids
Crystalline solids is the type of solids that are made up of crystals. When heated, they melt at a distinct temperature.
Amorphous solids are the type of solids that the particles are not arranged in a regular pattern. Amorphous solids also dont melt at distinct temperatures.
DESCRIBING LIQUIDS
A liquid has a definite volume, but no definite shape of its own.
The particles of liquids are almost always in contact with one another. However, the particles in a liquid are not fixed in place. They can move around one another.
This is the particle arrangement of a liquid.

Physical Properties of Liquid
Surface Tension is an inward force, or pull among the molecules in a liquid that brings the molecules on the surface closer together.
Viscosity is a liquid’s resistance to flowing.
DESCRIBING GASES
A gas has neither a definite volume nor a definite shape. This because the particles in a gas do not remain in contact with one another.
The particles of gas are moving all over the place, and they collide with one another as they fly all around.
This is the particle arrangement of gas.

Physical Properties of Gases
Since particles of gas move all over the place, the volume of gas is the same as the volume of its container.
TYPES OF ENERGY
Potential Energy is energy that is stored in an object due to its position or condition
Kinetic Energy is the energy an object has because of its motion.
Electrical Energy is generated by the movement of electrons from one point to another
Motion Energy is the energy stored in moving objects
Elastic Energy is energy stored in an object due to a force that temporarily changes its shape, such as squashing or stretching.
CHANGES OF STATE
Thermal Energy is the total kinetic and potential energy of all the particles in and object or substance.
You can increase the thermal energy by heating it.
Temperature is a measure of the average kinetic energy of the moving particles in an object or substance.
CHANGES OF STATE BETWEEN SOLID AND LIQUID
Melting point the temperature at which a substance changes from a solid to a liquid
The melting point of water is 0°C.
Freezing point is the temperature at which a liquid freezes.
CHANGES OF STATE BETWEEN LIQUID AND GAS
Vaporization the change of state from a liquid to a gas. This occurs when the particles in a liquid gain enough energy to move independently and away from each other. There are two main types of vaporization: boiling and evaporation.
Boiling point is the temperature at which a liquid boils.
Evaporation the process by which molecules at the surface of a liquid absorb enough energy to change to a gas.
while boiling occurs only at one temperature, evaporation can occur at all temperatures.
THE EFFECT OF PRESSURE
When you push on an object, you apply pressure. The pressure depends on the force you apply, and the area over which you apply the force.
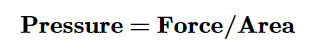
GAS TO A LIQUID
Condensation is the change in state from a gas to a liquid. It occurs when particles in a gas lose thermal energy to change state.
CHANGING STATE FROM A SOLID TO A GAS
Sublimation is the change in state from a solid directly to a gas without passing through the liquid state.
GAS BEHAVIOR
The pressure of a gas is the force of its outward push.→ FORCE
Force is measured in Newtons, over an area (in square meters) of the wall of the container.
Pressure is measured in units of pascals (PA), or kilopascals (kPA)
Temperature also affects the pressure of gas. When one heats a gas, its particles move faster, so the temperature increases
A greater force over the same area results in greater pressure
In general, when the temperature of a gas at a constant volume is increased, the pressure of the gas decreases. *
CHARLES’S LAW
A principle that describes the relationship between the temperature and volume of a gas at constant pressure.
BOYLE’S LAW
A principle that describes the relationship between the pressure and volume of a gas at constant pressure.
Boyle’s law describes situations in which the volume of a gas is changed. The pressure changes the opposite way.
INVERSELY PROPORTIONAL RELATIONSHIPS
When volume is low, pressure is high
When pressure is high, volume is low.
As the volume of gas increases at a constant temperature, the pressure of gas decreases at a different rate.
ENERGY, MOTION, FORCE, AND WORK
Energy is the ability to do work or cause change. Energy comes in many forms. Light, sound and electricity are all types of energy. Energy can also be transferred from place to place.
It takes energy for motion to occur. An object is at motion if its position changes relative to another object.
Motion the state in which one object’s distance from another is changing.
The relationship between energy and motion also involves forces.
Force a push or pull exerted on an object.
Work force exerted on an object that causes it to move.
WORK REQUIRES MOTION
No matter how much force you apply on something, if it doesn’t move, you are not doing any work to it.
The amount of work that one does depends on both the amount of force one exerts and the distance it moves.
CALCULTING WORK
The amount of work done on an object is calculated by multiplying force times distance
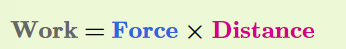
When force is measured in newtons and distance in meters, the SI unit of work is called a joule (J). One joule is the amount of work you do when you exert the force of 1 newton to move an object a distance of 1 meter.
WORK RELATED TO ENERGY AND POWER
Energy is measured in joules, the same units as work.
The time it takes to do work does not affect the amount of work you do on an object. But, something else—power— is affected.
Power is the rate at which one form of energy is transformed into another.
An object that has more power than another object does more work in the same amount of time. It can also mean doing the same amount of work in less time.
CALCULATING POWER
All one needs to know to calculate power is how much and how quickly work is being done.
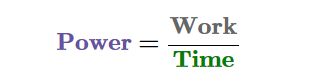
This formula can also be replaced with force x distance, and it would replace work. This depends on the question they ask.
When work is measured in joules and time in seconds, the SI unit of power is the watt (W).
1 WATT= 1 joule/seconds → 1 J/s
POWER AND ENERGY
Power is also the rate at which energy is transferred, or the amount of energy transferred in a unit of time.

 Knowt
Knowt