
Environmental Biology Final Study Guide 👩🏽🔬
What is Science?
Vocabulary Terms
Science: A philosophy used to answer questions about the natural world through observation and experimentation
Independent (Manipulated) Variable: Variable being changed, on the x axis
Dependent (Responding) Variable: This is the variable you measure (AKA what are you changing?), on the y axis
Scientific Method: Way of collecting evidence that supports or rejects a prediction
Controlled Experimentation: Tying to answer a question by changing one variable at a time; one thing must be changed; one thing must be measured
Control Group: Under “normal” conditions, used for comparison
Experimental Group(s): One variable is changed
Scientific Method
Steps of Scientific Method
Make an observation
Ask a question
Research
Make a Hypothesis
Develop a controlled experiment
Conduct the experiment; measure and record data
Analyze data
Draw Conclusion
Share your results and try again
Conclusions
Support hypothesis
Reject hypothesis
Leave the hypothesis inconclusive
Experimental Design
Independent Variable: Variable being changed
Dependent Variable: This is the variable you measure (AKA what are you changing?)
Controlled Experimentation: Tying to answer a question by changing one variable at a time; one thing must be changed; one thing must be measured
Control Group: Under “normal” conditions, used for comparison
Experimental Group(s): One variable is changed
Levels of Organization
Subatomic Particles
ex: protons, neutrons, electrons
Atoms
hydrogen, oxygen, carbon
Molecules
Two or more atoms chemically combined
ex: H20, C02
Macromolecules
Smaller molecules combined
ex: proteins, lipids, carbohydrates, nucleic acids
Organelles
Organs of the cell
ex: mitochondria, nucleus, lysosomes, etc….
Cells
Plant vs animal
Prokaryotic (no nucleus) and Eukaryotic (nucleus)
Tissues
Groups of similar cells performing similar functions
ex: lung, muscle, connective tissues
Organs
ex: lungs, pancreas, kidney
Organ System
Groups of organs working together
ex: reproductive, nervous, digestive
Organisms
Plants, Humans, Animals
Populations
Group of similar organisms, living in the same region
ex: school of fish, humans, murder of crows
Community
A bunch of populations in the same area
ex: Masters Campus
Ecosystem
Community + non living things (abiotic)
ex: Forest
Biomes
Ecosystems that have similar populations as well as environmental conditions; not necessarily near each other
ex: desert, tundra, rain forest
Biospheric
All the biomes
ex: earth
Solar System
Galaxy
Universe
Characteristics of Living Organisms
Living things share 8 basic characteristics
They are made of cells
They reproduce
They are based on a universal genetic code
They grow and develop
They use materials and energy
They respond to the environment
They maintain an internal balance (homeostasis)
They change over time
Biochemistry
Protons
The # of protons defines what type of element an atom is
The # of protons = the atomic number
Neutrons
Different atoms of the same element can have different number of neutrons; called isotopes
Electrons
Atoms can gain, lose, or share electrons
Atoms are electrically neutral because the # of protons = # of electrons
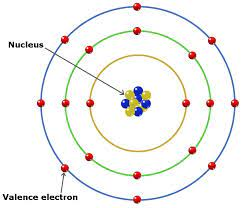
Structure of an Atom
Goal of most atoms is to have 8 valence electrons
Except hydrogen
Valence Electrons: Electrons in the outermost energy level (rings)
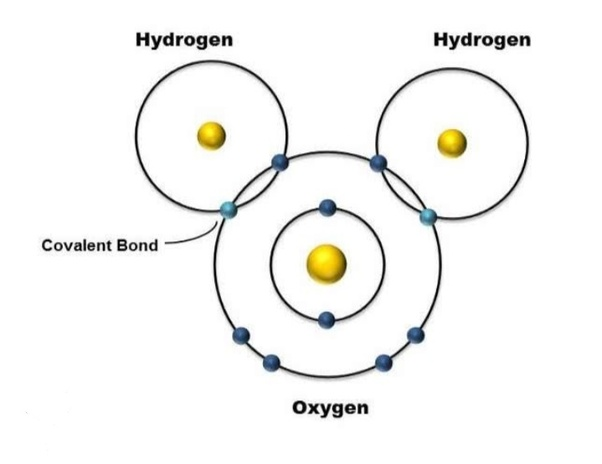
Covalent bonds atoms share electrons
Water is a polar molecule (it has a positive and negative side)
Covalent Compounds
Form when atoms share electrons
Properties of Water
Water is…..
Cohesive: Water molecules “stick” to other water molecules
ex: water on a penny
Adhesive: Water molecules “stick” to other substances
ex: The smaller tube having the most water
High Heat Capacity: Slow to heat and its slow to cool
ex: On a humid day, the sand gets hot but the ocean remains cold
Surface Tension: The molecules on the top of a water sample are attracted to the molecules beneath which creates a thin “net” holding the water together'
ex: bugs being able to walk on water
Polar: Allows water molecules to attract each other (through hydrogen bonding) and interact with other polar molecules and have a positive and negative side
Universal Solvent: Most ionic compounds will dissolve in water; most polar covalent compounds will dissolve in water
ex: Salt dissolving in water
Capillary Action: The ability of a liquid to flow in narrow spaces without the assistance of external forces like gravity.
ex: Water getting from roots to plants
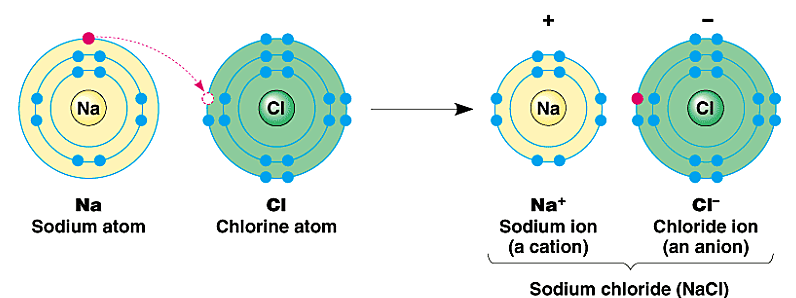
Ionic Bonds
Form when atoms transfer electrons
one atom has a (+) charge
one atom has a (-) charge
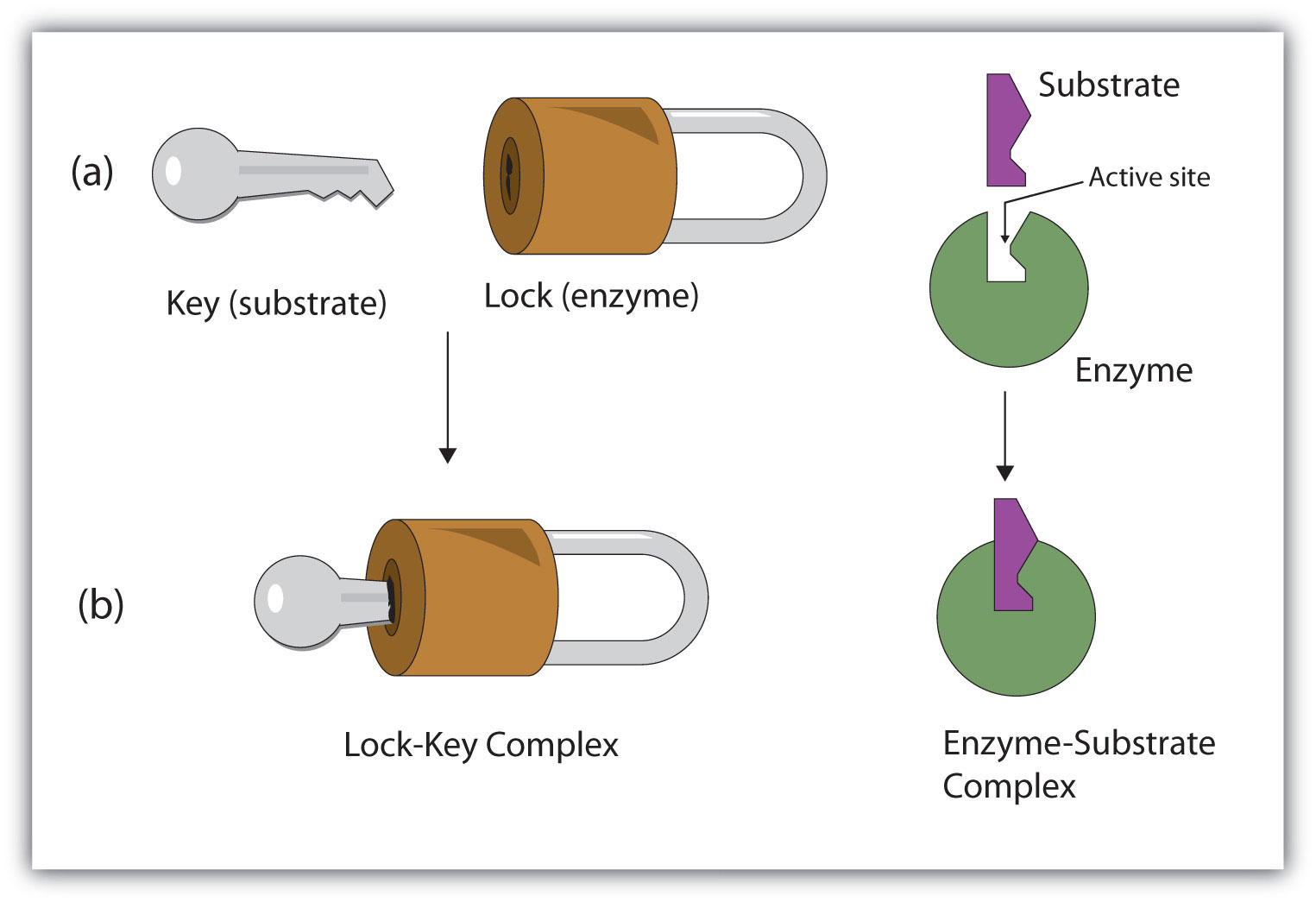
 Enzymes
Enzymes
Biological Catalysts
Proteins (proteins are made of amino acids)
Has its own unique 3D shapes
Has a different “R” group
Each enzyme is unique to a specific substrate
Lock (enzyme) + Key (substrate)
Enzymes can be denatured (change shape) by:
Change in temperature
Change in pH
Catalysts
Speeds up chemical reactions at a lower temperature
They are not consumed
Both Reactants and products
Reusable
Macromolecules
Carbohydrates
Composed of: Hydrogen, oxygen, and carbon (monosaccharides)
Monomers: Glucose, Fructose, and galactose
Examples: Sugar, starch cellulose
Function: Short Term Energy
Lipids:
Composed of: Carbon, oxygen, and hydrogen
Monomers: Glycerol and Fatty Acids
Examples: Oil, wax, glyceride
Function: Insulation, long term energy
Proteins
Composed of: Nitrogen, hydrogen, oxygen, carbon
Monomers: Amino acids
Examples: Enzymes, hormones
Function: Control rate of reactions and regulates cell processes, transports substances in and out of cells
Nucleic Acids
Composed of: Carbon, hydrogen, oxygen, nitrogen, phosphates
Monomers: Nucleotides
Examples: DNA and RNA
Function: Store and transfer genetic and hereditary information
Cell Organelles
Cell Types
Nucleus
Membrane-bound
Contains DNA
Shares genetics
Present in eukaryotic cells
Ribosome
Particles of RNA
Build/Synthesize proteins
Present in both prokaryotic and eukaryotic cells
Endoplasmic Reticulum (ER)
Rough ER has ribosomes attached to surface
Produce lipids, carbs, and proteins
Present in eukaryotic cells
Golgi Apparatus
Sort and package the proteins and lipids for storage or for transport out of the cell
Present in eukaryotic cells
Lysosomes
Contains enzymes
Breaksdown macromolecules
Present in eukaryotic (animal) cells
Vacuoles
Store materials and water
Smaller in animal cells, bigger in plant cells
Present in both prokaryotic and eukaryotic cells
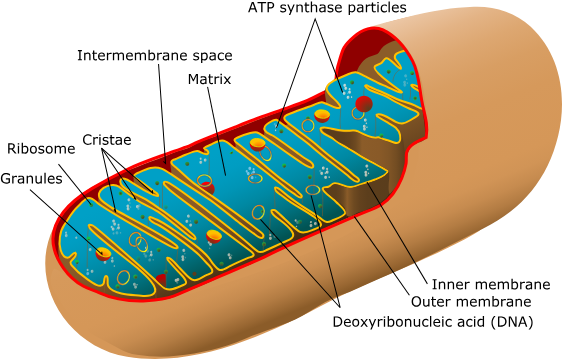
Mitochondria
“Powerhouse” of the cells
Converts chemical energy into a useable form
Respiration = usable energy
Present in eukaryotic cells'
Chloroplasts
Absorbs sunlight
Produces sugar
Present in eukaryotic plant cells
Cell Membrane
Regulates the passage of substances in and out of the cell
Present in prokaryotic cells and eukaryotic cells'
Cell Wall
Surrounds the cell membrane
Provides structure and support
Present in prokaryotic and plant cells
Cytoplasm
Jelly like substances that fill the cells
Other organelles “float” in it
Present in both prokaryotic and eukaryotic cells
Prokaryotes
No nucleus
No membrane-bound cells
Simple
DNA is “free floating”
Smaller than eukaryotic cells
ex: bacteria
Eukaryotic Cells
Eukaryotic cells have a membrane-bound nucleus
More complex than prokaryotes
Bigger than prokaryotes
ex: animal cells, human cells, plant cells
Cell Concepts
Membranes are fluid and flexible
Membranes can self-repair
Eukaryotic cells feature membrane bound organelles
Membrane proteins perform special functions
Plant Cell
Cell Wall
Cytoskeleton
Golgi Apparatus
Vacuole
Nucleus
Endoplasmic Reticulum
Ribosome
Chloroplast
Cell membrane
Mitochondria
Cytoplasm
ccccgevnmr
Animal Cell
Cell Membrane
Cytoplasm
Lysosomes
Mitochondria
Endoplasmic Reticulum
Ribosomes
Golgi Apparatus
Nucleus
cclmergn
Vocabulary
Cholesterol: A hydrophobic lipid molecule that changes the fluidity of the membrane
Phospholipid: Lipids with hydrophilic heads and hydrophobic tails that form two layers in the membrane and can move
Transport Proteins: Proteins that help carry substances across the membrane or allow molecules to pass through a channel
Glycolipid: Lipids with carbohydrate chains that serve as cell recognition, helps with cell communication
Glycoprotein: Proteins with carbohydrate chains that serve as cell recognition, helps with cell communication
Protein Channels: Provides safe passage for molecules (ions) that can’t go through the phospholipid bilayer
Cytoskeleton Filaments: Long protein chains that help the cell hold its shape, organelles and other large molecules can travel along these chains like super highways in the cell
Phospholipid Head: Hydrophilic, polar
Phospholipid Tail: Hydrophobic, non-polar
Cell Membrane
Passive Transport (Require NO energy)
Diffusion
When particles flow from high concentration to low concentration
Across cell membranes
Non polar molecules
ex: The smell of hand lotion
Facilitated Diffusion
Particles move from up to down through protein channels
ex: VIP line
Osmosis
Diffusion of water through a semipermeable membrane
Moves through aquaporins
Water moves from an area of high concentration to low concentration
Goal is to balance things out on both sides of the barrier
Active Transport (Requires ATP)
Requires energy
Moves from low to high concentration
Uses protein pumps; pumps change shape to fit particles
Exocytosis
Process used by cells to move substances out of the cell
Vesicles: Contain the substances that are being moved
Endocytosis
Process where the cell takes in materials from the outside environment
Protein Pumps
Help move molecules across the membrane across the gradient
Cell Theory
All living organisms are made from cells
Cell are the basic unit of life
All cells come from other cells
Hypotonic
When comparing two solutions, the solution with the lower amount of solute
Cells swell, then lead to potential bursting
Hypertonic
When comparing two solutions, the solution with the higher amount of solute is called hypertonic
Cells shrink
Isotonic
A solution that has the same concentration of solutes as another solution, leading to no movement of water
Cytolysis
A process that occurs when a cell swells and bursts due to too much water in a hypotonic solution
Can be prevented by the cell wall (in plant cells) by providing structural support
Plasmolysis
A process that occurs when a plant cell loses water and shrinks away from its cell wall due to being placed in a hypertonic solution
Photosynthesis
Chlorophyll and Contrasts
Sunlight is “white” light- actually a mixture of different wavelengths
Photosynthetic organisms capture light energy from sunlight with pigments
Light energy from the sun must be captured for photosynthesis
Pigments: light-absorbing compounds
Chloroplasts: Organelle where photosynthesis takes place
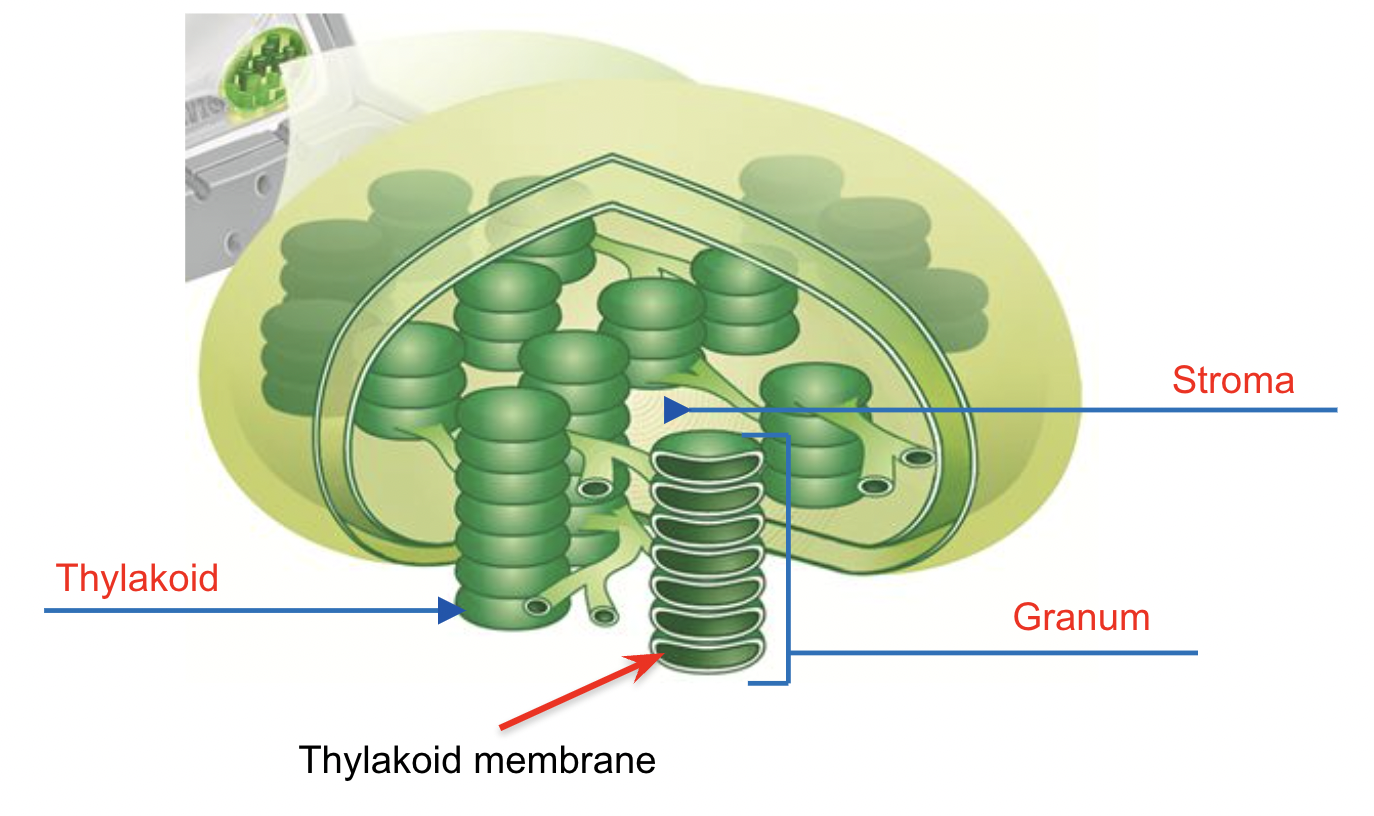
Chloroplast Structure
Chloroplast is stored in the thylakoid membranes
Electron Carriers
A compound that can accept a pair of high energy electrons and transfer them, a long with most of their energy.
A compound called NAPD+ acts as an electron carrier by accepting 2 high energy electrons and 1 hydrogen ion
NADPH can carry the high-energy electrons that were produced by light absorption in the chlorophyll to chemical reactions elsewhere in the cell
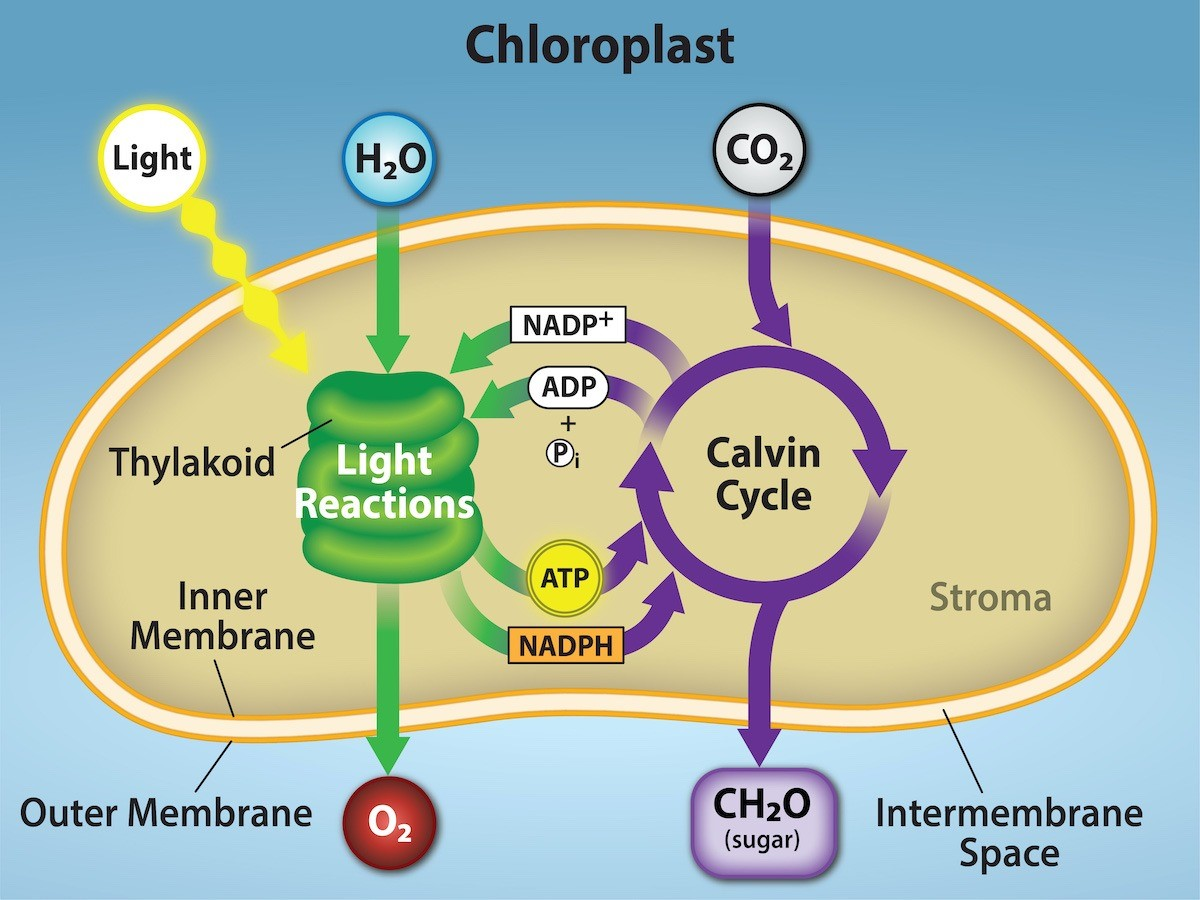
Overview of Photosynthesis
Photosynthesis uses the energy of sunlight to convert water and carbon dioxide (low energy reactants) into high energy sugars and oxygen (products)
Chemical Equation: 6CO2 + 6H20 → C6H120O6 + 6O2
Autotrophs do photosynthesis
Photosynthesis and Light
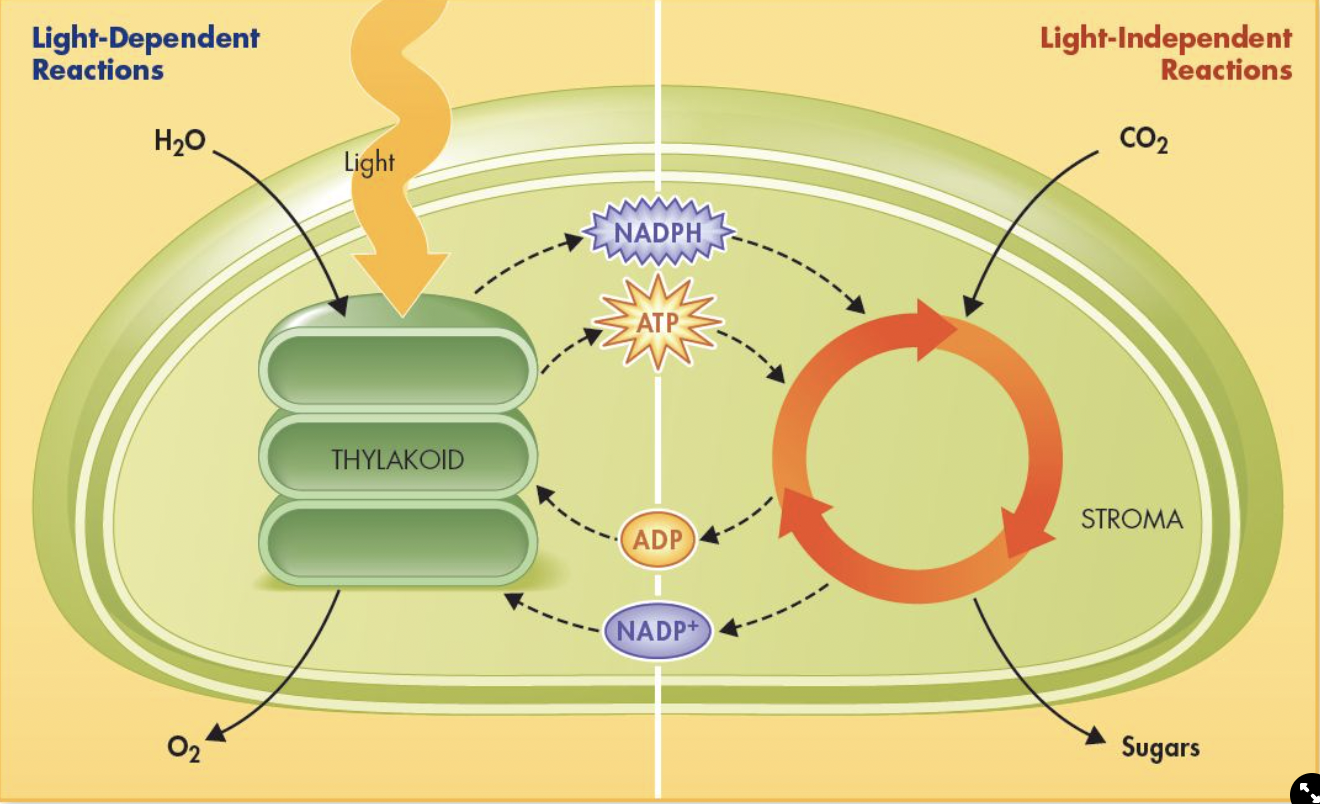
Light-dependent reactions
Light-independent reactions
 oxygen comes from the water,
oxygen comes from the water,
Light Dependent Reactions
Occur in the thylakoid membranes of chloroplasts
Inputs: H20, Light, ADP, NADP+
Outputs: O2, ATP, NADPH
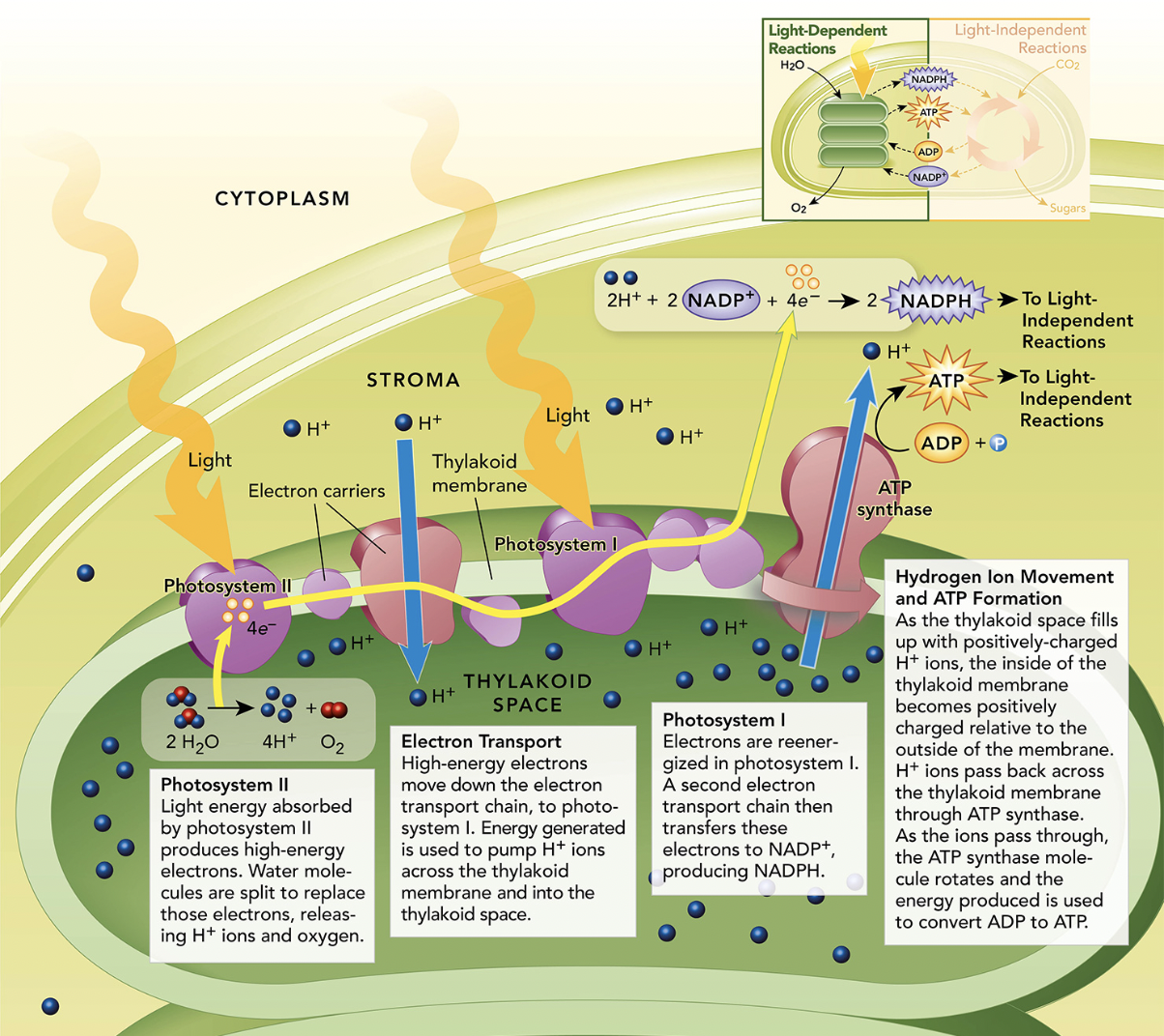
Steps
The sun strikes an electron that is inside PSII. The electron then gets excited
The electron has too much energy so it can’t stay in PSII, so it goes through the electron transport chain. As the electron goes through the ETC, hydrogen and oxygen atoms are going to be released into the thylakoid
The hydrogen that were released during the ETC, are going to be pushed down through ATP synthase and into the stroma. The hydrogen ions that are in the stroma now, turn the ADP into ATP
Eventually, sunlight will excite the electron that is now in PSI and goes through the second ETC. That electron then converts NADP+ into NADPH
Light Independent (Calvin Cycle) Reactions
Occurs in the stroma of the chloroplast
Inputs: CO2, ATP, NADPH
Outputs: Glucose (C6H12O6), ADP, NADP+
Cellular Respiration
Overview of Cellular Respiration
Cellular respiration is a process of energy conversion that releases energy from food in the presence of oxygen
Everything
Cellular Respiration Chemical Equation
In symbols:
6O2 + C6H12O6 → 6CO2 + 6H20 + ATP
ETC Glycolysis Krebs ETC (Majority come from ETC)
In Words:
Oxygen + Glucose → Carbon dioxide + Water + Energy
Glycolysis
Where does it occur?: Cytoplasm
Inputs: ATP, 1 Glucose, NAD+, ADP
Outputs: 2 pyruvic acid, 4 ATP (2 net)
Where do the outputs go?: The pyruvic acid goes to Krebs Cycle, ATP is used by the cell, NADH goes to ETC
Doesn’t require oxygen (anaerobic), quick energy
Krebs Cycle
Where does it occur?: Mitochondrial matrix
Inputs: 2 pyruvic acid, NAD+, FAD, ADP
Outputs: 6 CO2, 8 NADH, 2 ATP, 2 FADH2
Where do the outputs go?: NADH and FADH2 got to ETC, ATP gets used by the cell, CO2 diffuses out and you exhale
Electron Transport Chain
Where does it occur?: Inner Membrane of the Mitochondria
Inputs: NADH, FADH2, O2, ADP
Outputs: 6H2O, 34 ATP, NAD+, FAD
Where do the outputs go?: H2O is used by cells and leaves when exhaled, 34 ATP are used by the cell
Produces a lot of ATP used by the body
Fermentation
In the absence of oxygen, fermentation releases energy from food molecules by producing ATP
Glycolysis must occur first
Lactic Acid Fermentation Equation: Pyruvic Acid + NADH → Lactic Acid + NAD+
Alcoholic Fermentation Equation: Pyruvic Acid + NADH → Alcohol + CO2 + NAD+
Steps of Cellular Respiration
In Glycolysis, glucose molecules are split into two pyruvates
In the Krebs Cycle, the pyruvate molecules from glycolysis go to the mitochondrial matrix to find Coenzyme A. In the presence of NAD+, pyruvate gets attached to Coenzyme A nad is turned into acetyl-CoA
In the Electron Transport Chain, electrons flow through the electron transport chain, causing protons to be pumped from the matrix to the intermembrane space
Comparing Photosynthesis and Cellular Respiration
Photosynthesis “deposits” energy
Cellular Respiration “withdraws” energy
The equations for photosynthesis and cellular respiration are the reverse of each other
The products of one are the reactants of the other
Photosynthesis removes carbon dioxide from the atmosphere, and cellular respiration puts it back
Photosynthesis releases oxygen into the atmosphere, and cellular respiration uses that oxygen to release energy from food
Cell Cycle
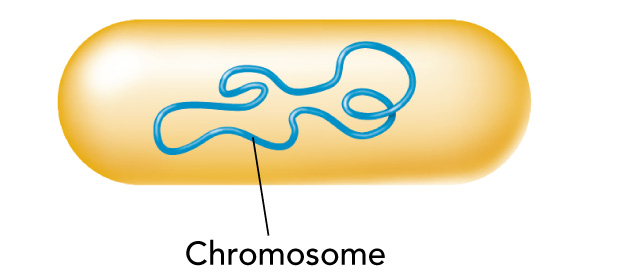
Prokaryotic Cells
Most prokaryotes contain a single circular DNA chromosome
Eukaryotic Chromosomes
Eukaryotic cells have much more DNA than prokaryotes have and contain multiple chromosomes.
Complex DNA and protein is referred to as chromatin
Mitosis Phases
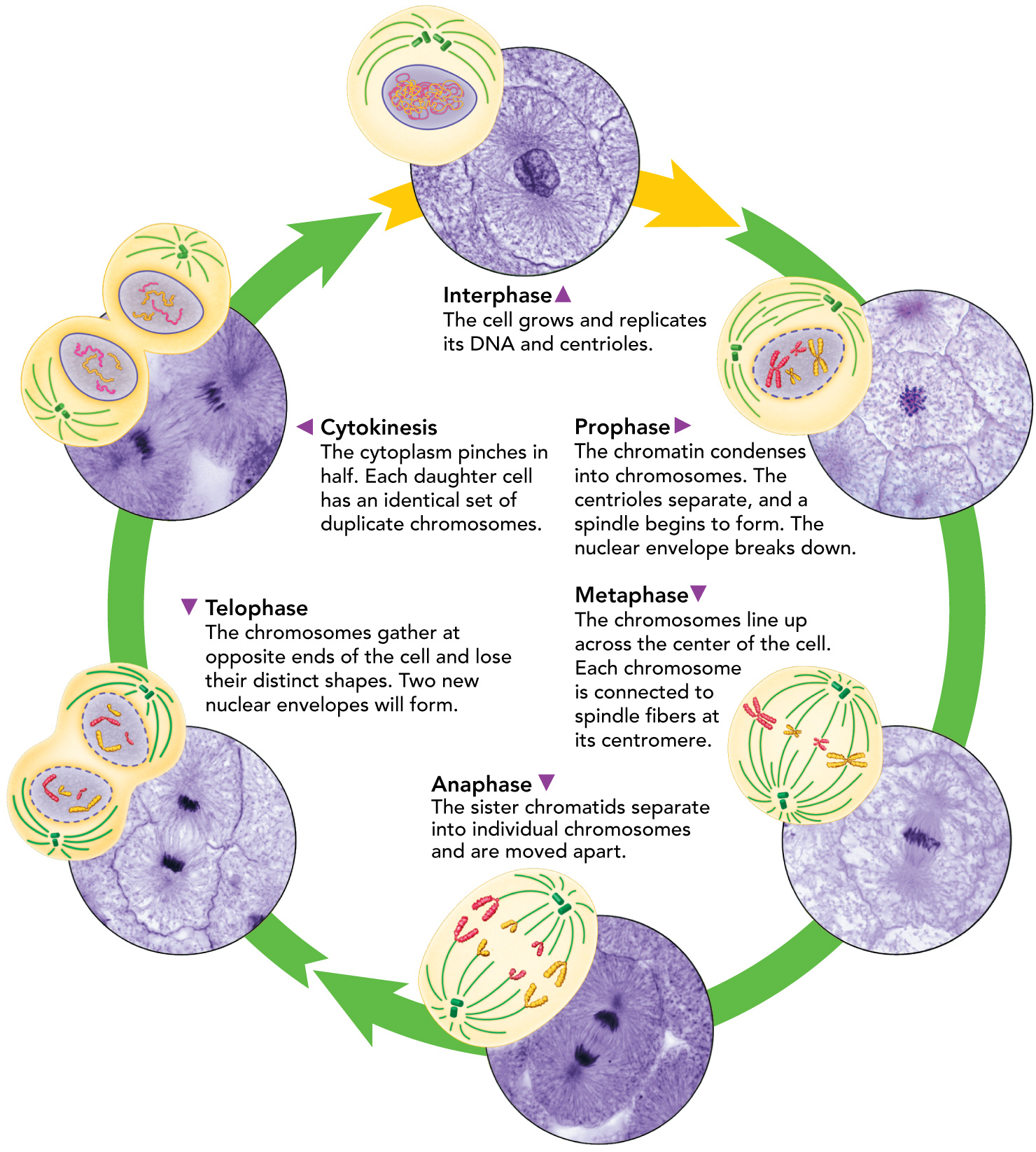
Prophase
Metaphase
Anaphase
Telophase
Cytokinesis
Cell Cycle
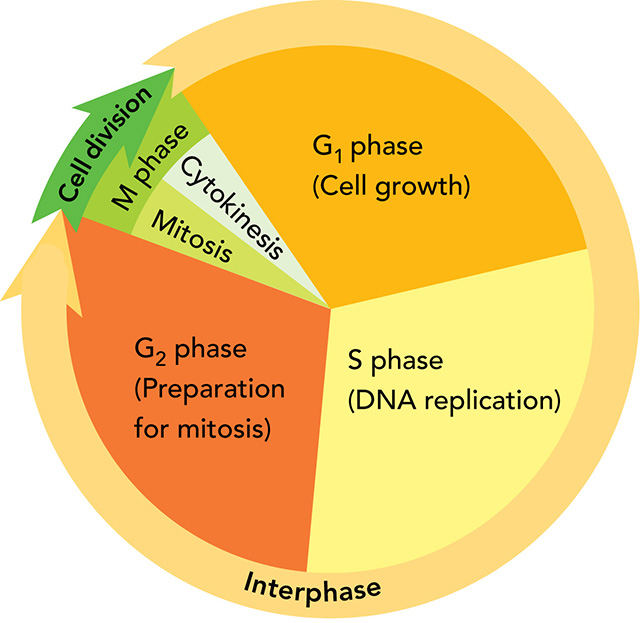
During the cell cycle, a cell grows, prepares for division, and then divides to form daughter cells. Each daughter cell then moves into a new cell cycle of activity, growth, and division.
Eukaryotic Cell Cycle
There are four stages: G1, S, G2, and M
In eukaryotes, cell division occurs in two main stages. The first stage of the process (the division of the nucleus) is called mitosis. The second stage (the division of the cytoplasm) is called cytokinesis
Interphase Phases
G1 (Cell Growth)
Cells do their most growing
Cells increase in size and synthesize new proteins and organelles
The G stands for “gap”
S (DNA Replication)
Follows G1
New DNA is synthesized as the chromosomes are replicated
By the end, cells contain twice as much DNA as it did at the beginning of the phase
S stands for “synthesis”
G2 (Preparing for Cell Division)
The shortest phase
Many of the organelles and molecules required for cell division are produced
Occurs after DNA replication is completed
M Phase (Cell Division)
Produces 2 daughter cells
It includes mitosis and cytokinesis
Follows interphase
Mitosis
Interphase
Period of the cell cycle between cell divisions in which the cell grows
Divided into 4 stages: G1, S, G2, and M phase
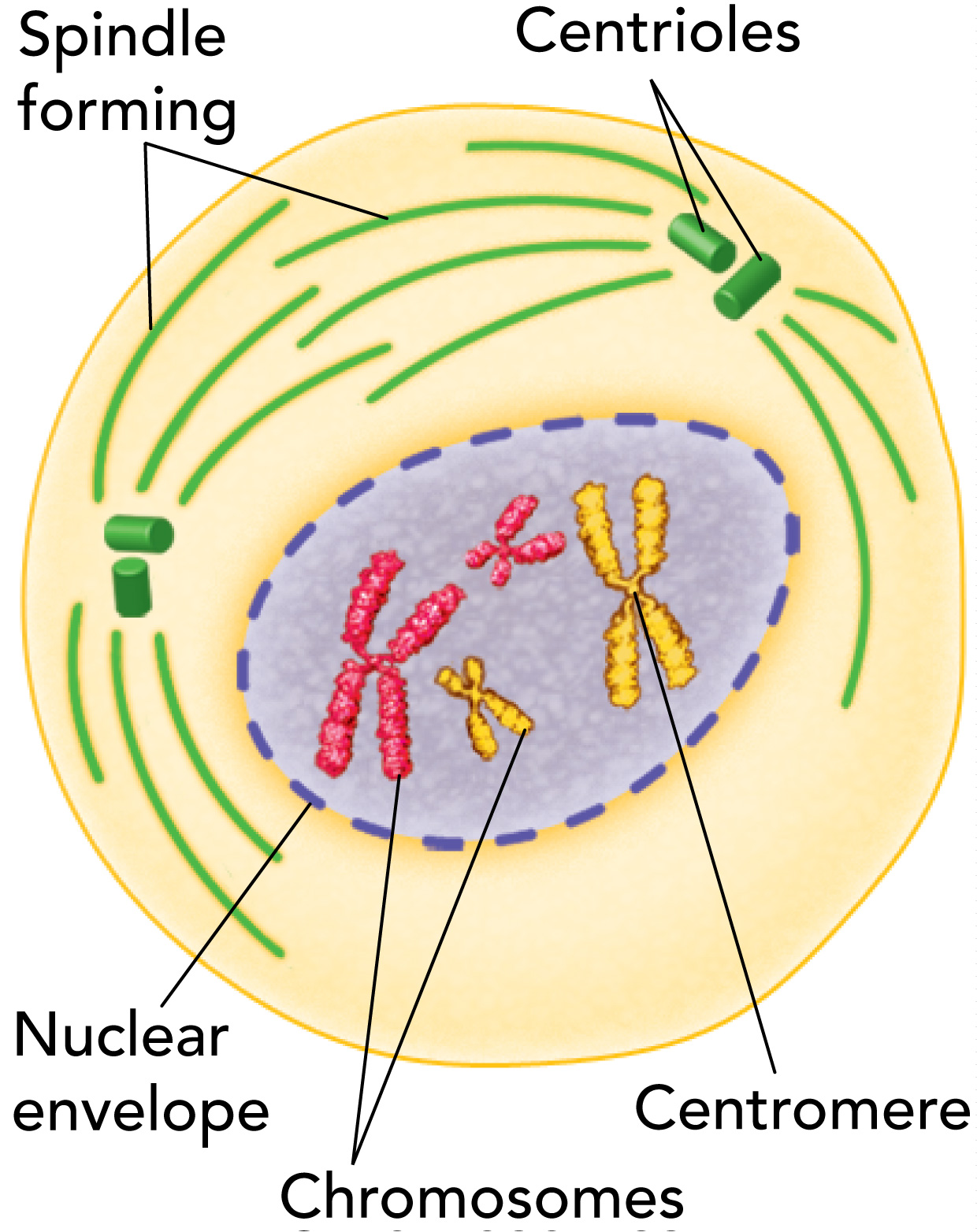
Prophase
The longest phase and may take up to half ot the total time required to complete mitosis
During prophase, the genetic material inside the nucleus condenses and the duplicated chromosomes become visible
Outside the nucleus, a spindle starts to form
A spindle helps separate the duplicated chromosomes
Each duplicated chromosome condenses to appear as two thick strands known as sister chromatids attached at a centromere
Metaphase
The shortest phase
During metaphase, the centromeres of the duplicated chromosomes line up across the center of the cell
Spindle fibers connect the centromere of each chromosome to the two poles of the spindle
At the end, the cell is ready to separate the sister chromatids
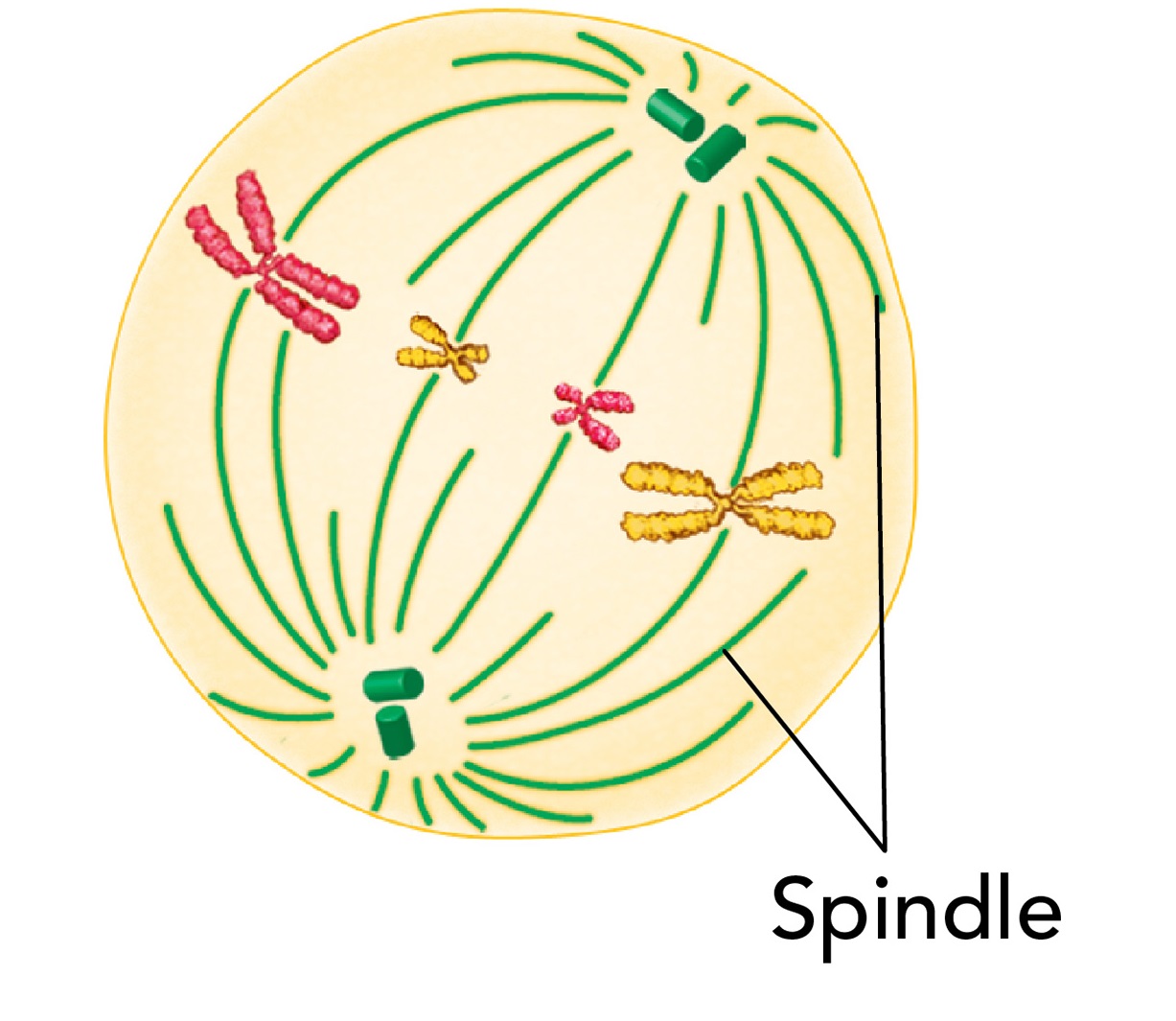
Anaphase
Begins when sister chromatids suddenly separate and begin to move apart
During anaphase, the chromosomes separate and move along spindle fibers to opposite ends of the cell.
When anaphase begins, each sister chromatid turns into an individual chromosome
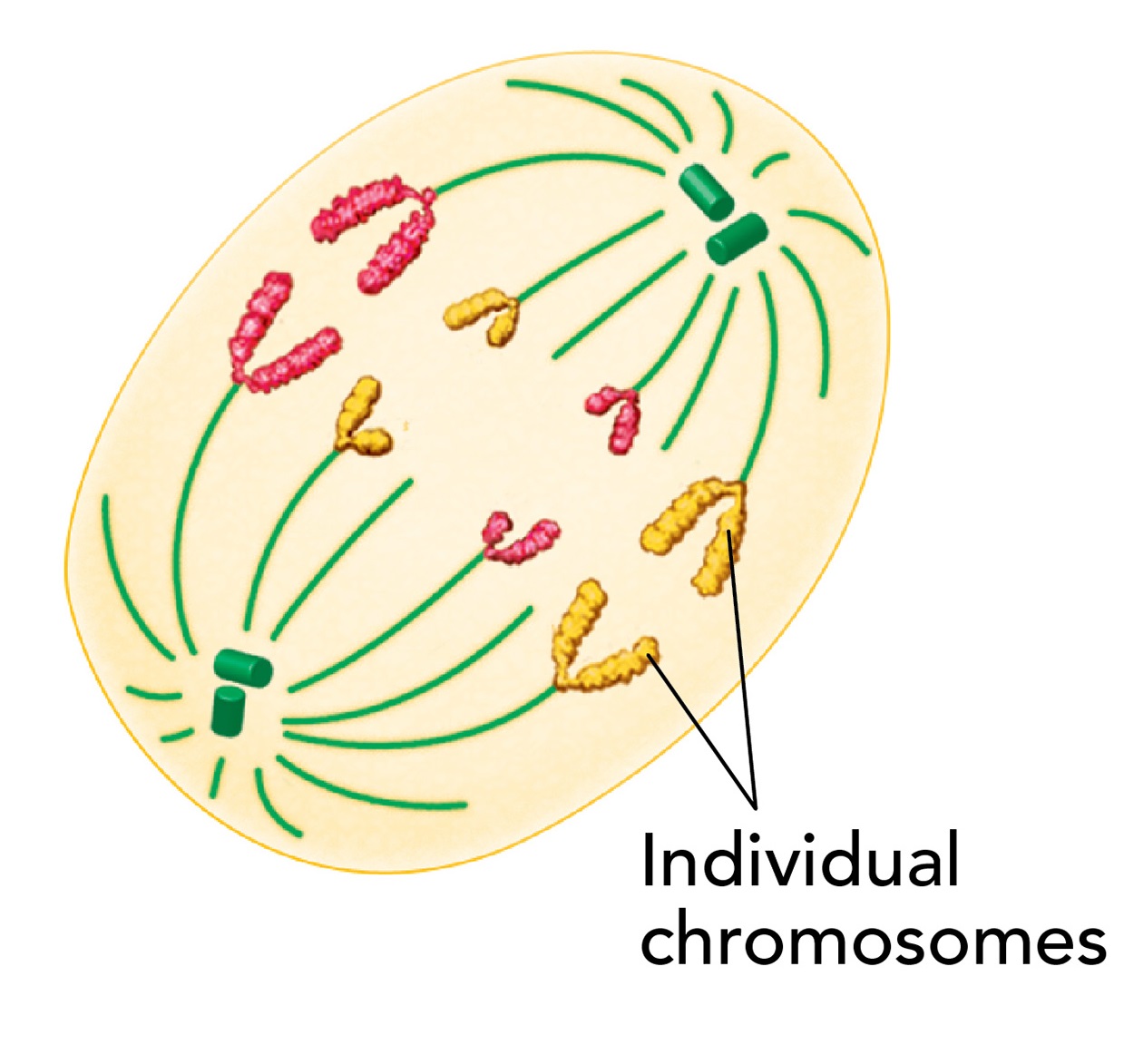
Telophase
During telophase, the chromosomes, which were distinct and condensed begin to spread out into a tangle of chromatin
A nuclear envelope reforms around each cluster of chromosomes, and gradually a nucleolus becomes visible in each daughter nucleus
Mitosis is completed, but the process of cell division has one more step
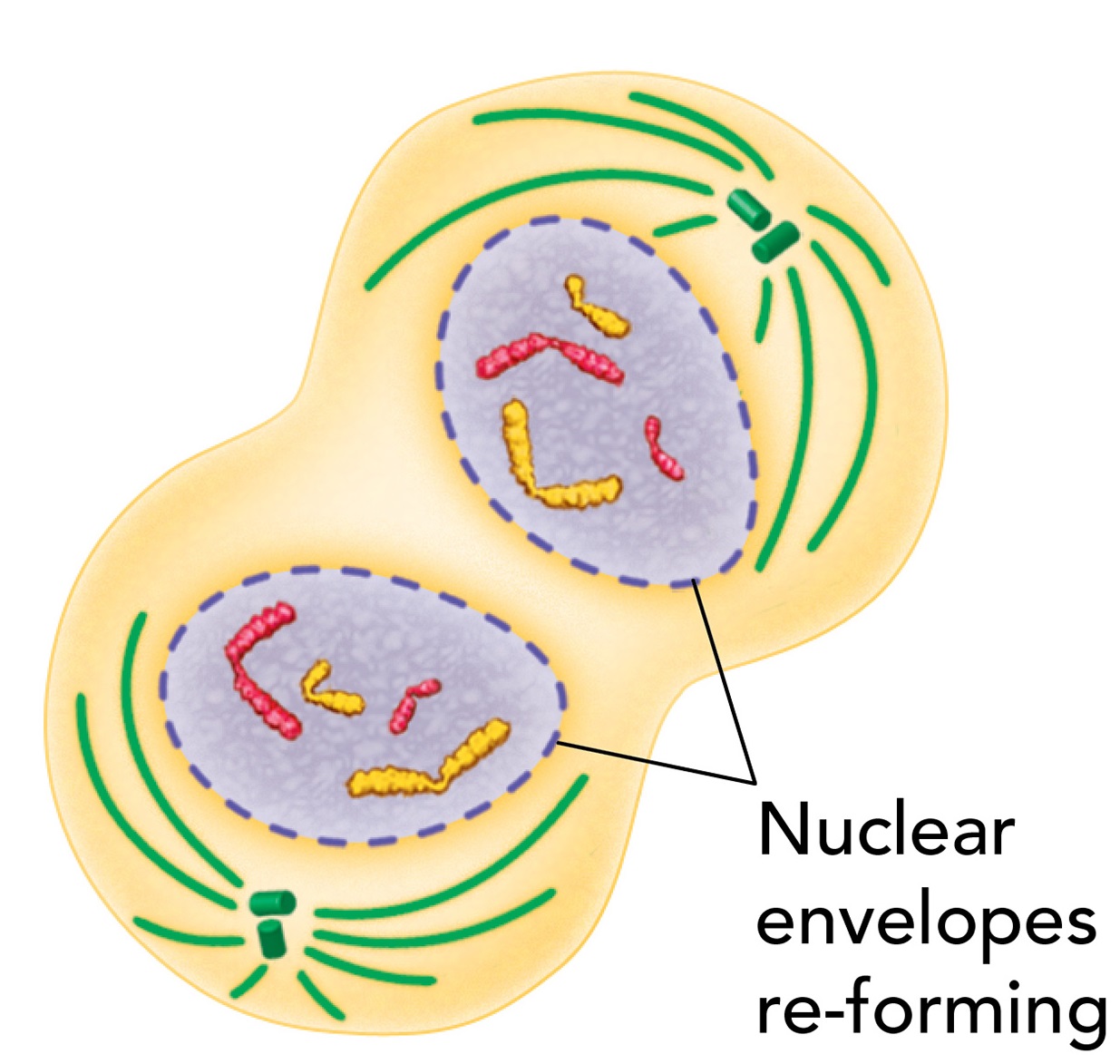
Cytokinesis
Cytokinesis is the division of the cytoplasm to form two separate cells
Cytokinesis completes the process of cell division by dividing one cell into two
Cytokinesis in Animal Cells
For most animal cells, the cell membrane is drawn inward until the cytoplasm is pinched into two nearly equal parts
Cytokinesis in Plant Cells
A structure known as the cell plate forms halfway between the divided nuclei.
The cell plate gradually develops into cell membranes that separate the two daughter cells.
Regulating the Cell Cycle
Cancer results in uncontrolled cell growth and division.
Rapid growing cancer cells can be targeted by radiation or chemotherapy or by surgically removing the tumor
Mitosis Visual Summary
DNA & Genetics
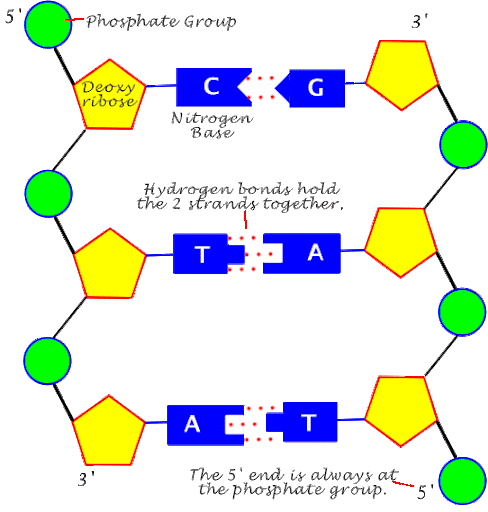
DNA
There are 4 Nitrogen Bases of DNA:
Adenine
Thymine
Guanine
Cytosine
The bases are connected through hydrogen bonds
Structure:
Punnett Squares
Punnett squares use mathematical probability to help predict the genotype and phenotype combinations in genetic crosses
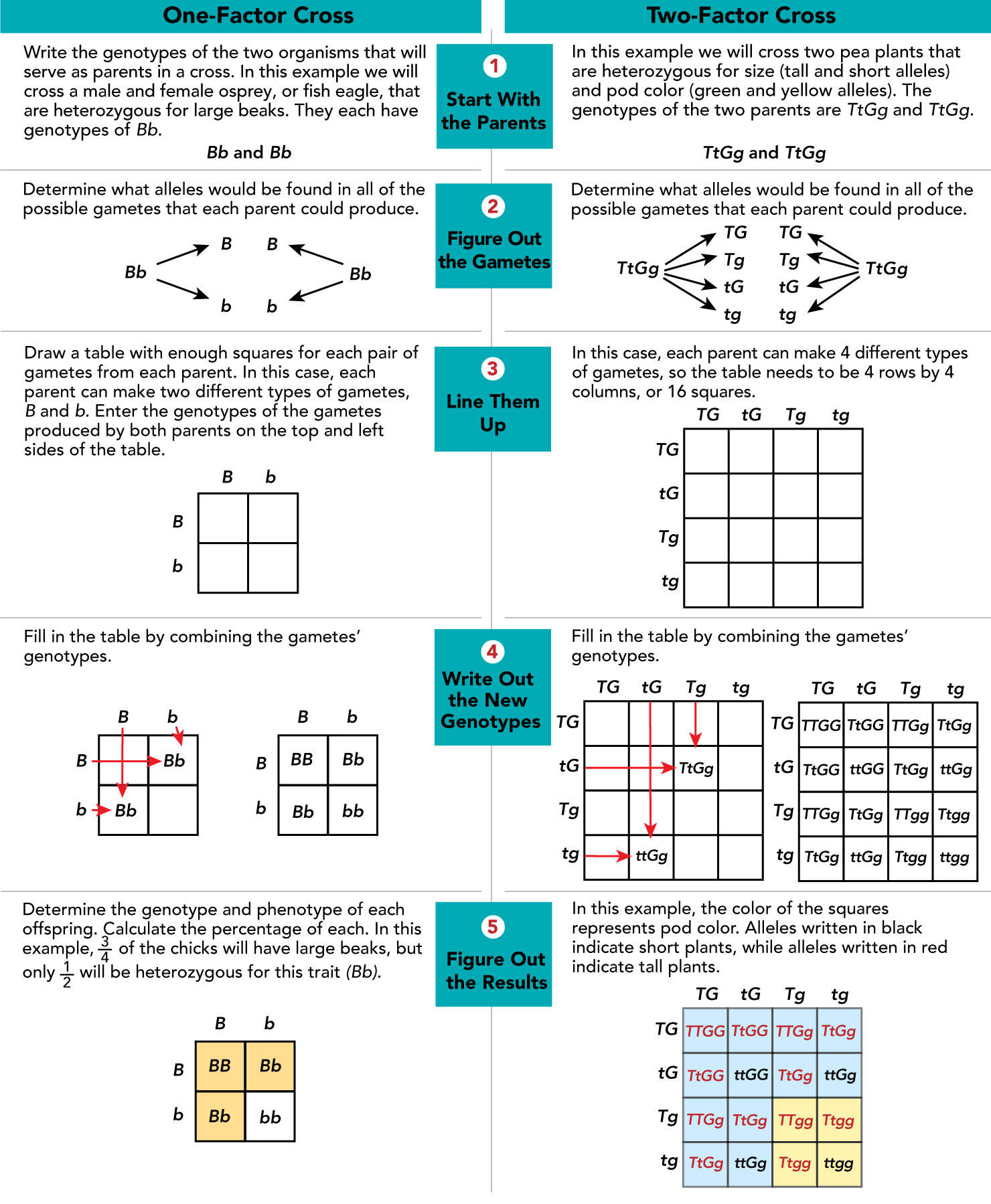
Summary of Mendel’s Principles
Mendel’s principles of heredity, observed through patterns of inheritance, form the basis of modern genetics
Mendel’s basic principles of inheritance can be used to study the inheritance of human traits and genetic disorders such as cystic fibrosis
2 alleles/gene
Alleles segregate independently during the formation of sex cells *gametes) and are passed on to an offspring independently
Mendel’s Principles of Heredity
The inheritance of biological characteristics is determined by individual units called genes, which are passed from parents to offspring
Where two or more forms (alleles) of the gene for a single trait exist, some alleles may be dominant and others may be recessive
In most sexually reproducing organisms, each adult has two copies of each gene- one from each parent. These genes segregate from each other when gametes are formed
Alleles for different genes usually segregate independently of each other
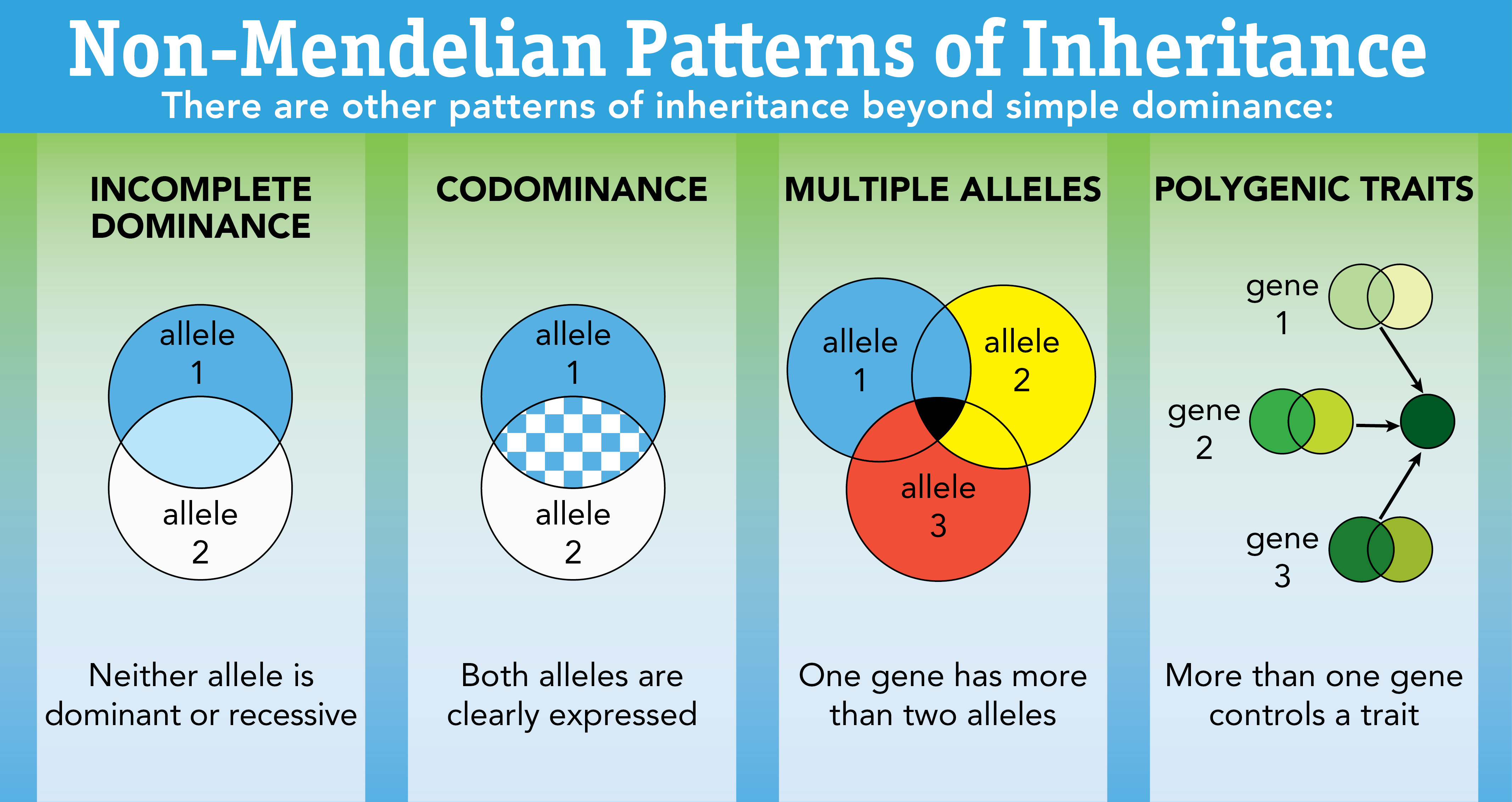
Other Patterns of Inheritance
Some alleles are neither dominant nor recessive
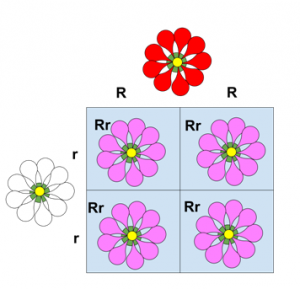
Incomplete dominance: One allele is completely dominant over another (there is a mix)
Ex: The flower is mixed
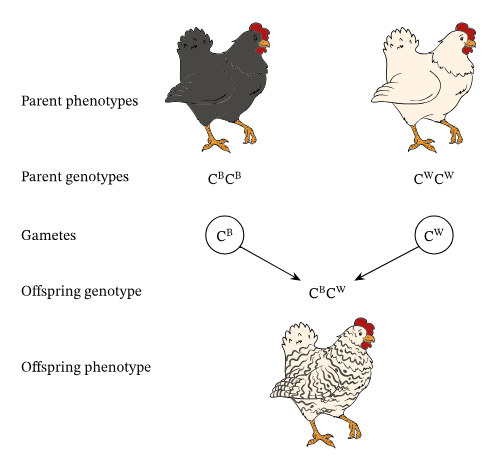
Codominance: The phenotypes for both alleles are clearly expressed
Ex: Chicken feather color, human protein controlling blood cholesterol levels
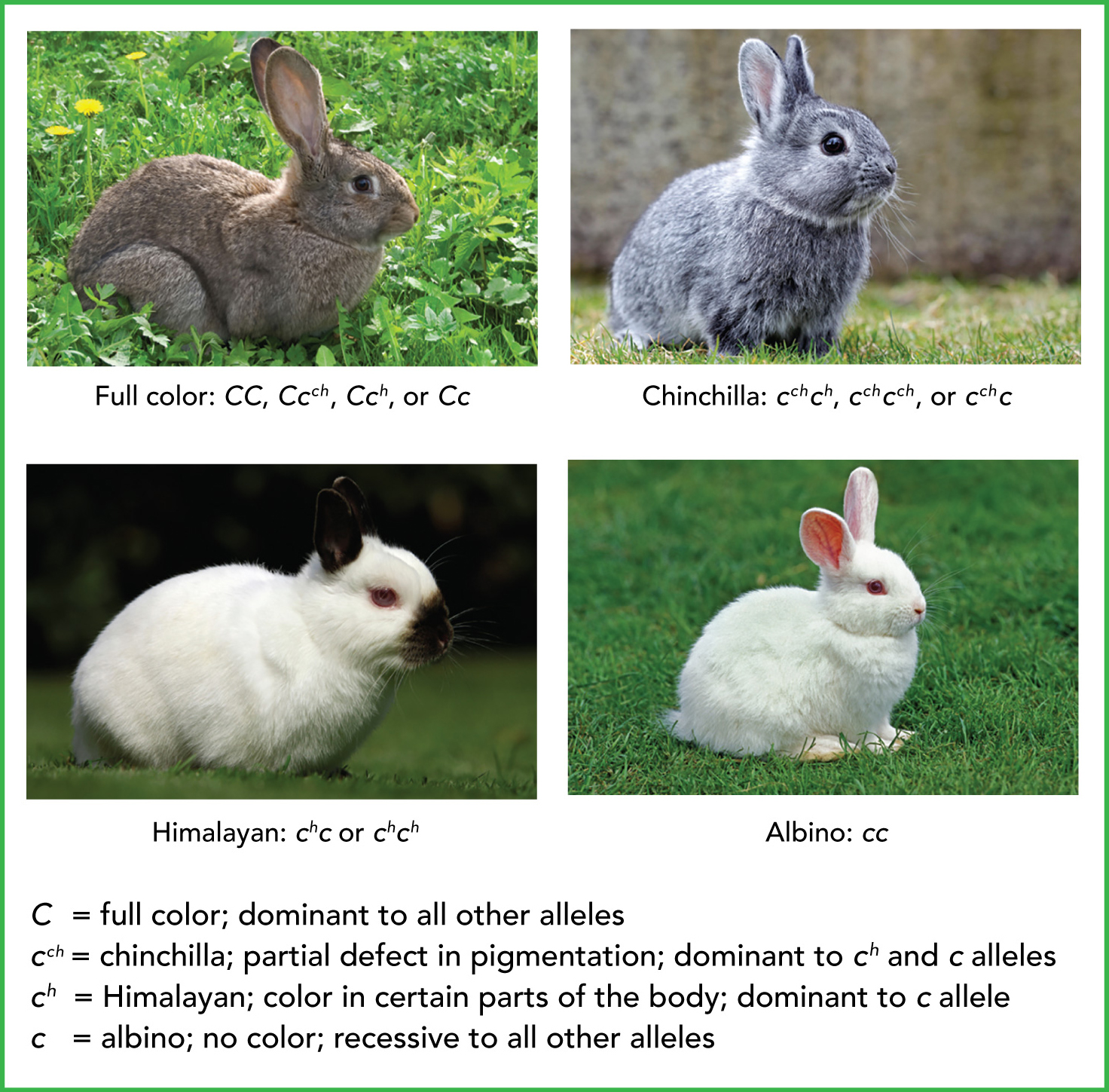
Multiple Alleles: One gene has more than two alleles
Ex: Human blood, fur types, fur color in rabbits
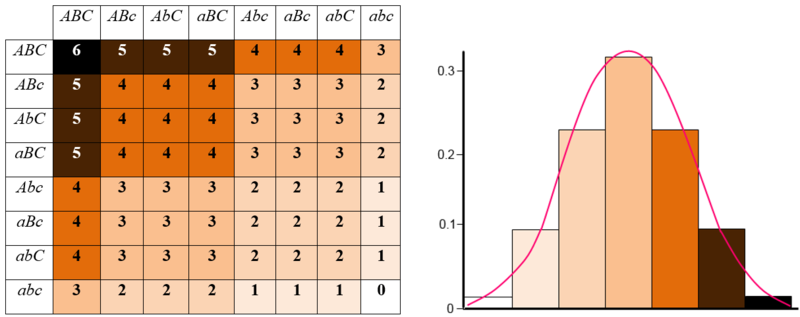
Polygenic Traits: Many traits are produced by the interaction of several genes
Ex: Eye color in fruit flies, coat color in dogs
Trais typically show a wide variety of phenotypes
Based on incomplete dominance
Are often influenced by the environment
Evolution
Who is Darwin?
Father of evolution developed theory of natural selection
Evolution: A change in allele frequencies within a population over time
Natural Selection: Process by which organisms are most suited to their environment survive and reproduce successfully
Things to Know How to Draw
Chloroplasts
Mitochondria
Prophase, Metaphase, anaphase, and telophase
DNA formatting
Environmental Biology Final Study Guide 👩🏽🔬
What is Science?
Vocabulary Terms
Science: A philosophy used to answer questions about the natural world through observation and experimentation
Independent (Manipulated) Variable: Variable being changed, on the x axis
Dependent (Responding) Variable: This is the variable you measure (AKA what are you changing?), on the y axis
Scientific Method: Way of collecting evidence that supports or rejects a prediction
Controlled Experimentation: Tying to answer a question by changing one variable at a time; one thing must be changed; one thing must be measured
Control Group: Under “normal” conditions, used for comparison
Experimental Group(s): One variable is changed
Scientific Method
Steps of Scientific Method
Make an observation
Ask a question
Research
Make a Hypothesis
Develop a controlled experiment
Conduct the experiment; measure and record data
Analyze data
Draw Conclusion
Share your results and try again
Conclusions
Support hypothesis
Reject hypothesis
Leave the hypothesis inconclusive
Experimental Design
Independent Variable: Variable being changed
Dependent Variable: This is the variable you measure (AKA what are you changing?)
Controlled Experimentation: Tying to answer a question by changing one variable at a time; one thing must be changed; one thing must be measured
Control Group: Under “normal” conditions, used for comparison
Experimental Group(s): One variable is changed
Levels of Organization
Subatomic Particles
ex: protons, neutrons, electrons
Atoms
hydrogen, oxygen, carbon
Molecules
Two or more atoms chemically combined
ex: H20, C02
Macromolecules
Smaller molecules combined
ex: proteins, lipids, carbohydrates, nucleic acids
Organelles
Organs of the cell
ex: mitochondria, nucleus, lysosomes, etc….
Cells
Plant vs animal
Prokaryotic (no nucleus) and Eukaryotic (nucleus)
Tissues
Groups of similar cells performing similar functions
ex: lung, muscle, connective tissues
Organs
ex: lungs, pancreas, kidney
Organ System
Groups of organs working together
ex: reproductive, nervous, digestive
Organisms
Plants, Humans, Animals
Populations
Group of similar organisms, living in the same region
ex: school of fish, humans, murder of crows
Community
A bunch of populations in the same area
ex: Masters Campus
Ecosystem
Community + non living things (abiotic)
ex: Forest
Biomes
Ecosystems that have similar populations as well as environmental conditions; not necessarily near each other
ex: desert, tundra, rain forest
Biospheric
All the biomes
ex: earth
Solar System
Galaxy
Universe
Characteristics of Living Organisms
Living things share 8 basic characteristics
They are made of cells
They reproduce
They are based on a universal genetic code
They grow and develop
They use materials and energy
They respond to the environment
They maintain an internal balance (homeostasis)
They change over time
Biochemistry
Protons
The # of protons defines what type of element an atom is
The # of protons = the atomic number
Neutrons
Different atoms of the same element can have different number of neutrons; called isotopes
Electrons
Atoms can gain, lose, or share electrons
Atoms are electrically neutral because the # of protons = # of electrons
Structure of an Atom
Goal of most atoms is to have 8 valence electrons
Except hydrogen
Valence Electrons: Electrons in the outermost energy level (rings)
Covalent bonds atoms share electrons
Water is a polar molecule (it has a positive and negative side)

Covalent Compounds
Form when atoms share electrons

Properties of Water
Water is…..
Cohesive: Water molecules “stick” to other water molecules
ex: water on a penny
Adhesive: Water molecules “stick” to other substances
ex: The smaller tube having the most water
High Heat Capacity: Slow to heat and its slow to cool
ex: On a humid day, the sand gets hot but the ocean remains cold
Surface Tension: The molecules on the top of a water sample are attracted to the molecules beneath which creates a thin “net” holding the water together'
ex: bugs being able to walk on water
Polar: Allows water molecules to attract each other (through hydrogen bonding) and interact with other polar molecules and have a positive and negative side
Universal Solvent: Most ionic compounds will dissolve in water; most polar covalent compounds will dissolve in water
ex: Salt dissolving in water
Capillary Action: The ability of a liquid to flow in narrow spaces without the assistance of external forces like gravity.
ex: Water getting from roots to plants
Ionic Bonds
Form when atoms transfer electrons
one atom has a (+) charge
one atom has a (-) charge
 Enzymes
Enzymes
Biological Catalysts
Proteins (proteins are made of amino acids)
Has its own unique 3D shapes
Has a different “R” group
Each enzyme is unique to a specific substrate
Lock (enzyme) + Key (substrate)
Enzymes can be denatured (change shape) by:
Change in temperature
Change in pH

Catalysts
Speeds up chemical reactions at a lower temperature
They are not consumed
Both Reactants and products
Reusable
Macromolecules
Carbohydrates
Composed of: Hydrogen, oxygen, and carbon (monosaccharides)
Monomers: Glucose, Fructose, and galactose
Examples: Sugar, starch cellulose
Function: Short Term Energy
Lipids:
Composed of: Carbon, oxygen, and hydrogen
Monomers: Glycerol and Fatty Acids
Examples: Oil, wax, glyceride
Function: Insulation, long term energy
Proteins
Composed of: Nitrogen, hydrogen, oxygen, carbon
Monomers: Amino acids
Examples: Enzymes, hormones
Function: Control rate of reactions and regulates cell processes, transports substances in and out of cells
Nucleic Acids
Composed of: Carbon, hydrogen, oxygen, nitrogen, phosphates
Monomers: Nucleotides
Examples: DNA and RNA
Function: Store and transfer genetic and hereditary information
Cell Organelles
Cell Types
Nucleus
Membrane-bound
Contains DNA
Shares genetics
Present in eukaryotic cells
Ribosome
Particles of RNA
Build/Synthesize proteins
Present in both prokaryotic and eukaryotic cells
Endoplasmic Reticulum (ER)
Rough ER has ribosomes attached to surface
Produce lipids, carbs, and proteins
Present in eukaryotic cells
Golgi Apparatus
Sort and package the proteins and lipids for storage or for transport out of the cell
Present in eukaryotic cells
Lysosomes
Contains enzymes
Breaksdown macromolecules
Present in eukaryotic (animal) cells
Vacuoles
Store materials and water
Smaller in animal cells, bigger in plant cells
Present in both prokaryotic and eukaryotic cells
Mitochondria
“Powerhouse” of the cells
Converts chemical energy into a useable form
Respiration = usable energy
Present in eukaryotic cells'
Chloroplasts
Absorbs sunlight
Produces sugar
Present in eukaryotic plant cells
Cell Membrane
Regulates the passage of substances in and out of the cell
Present in prokaryotic cells and eukaryotic cells'
Cell Wall
Surrounds the cell membrane
Provides structure and support
Present in prokaryotic and plant cells
Cytoplasm
Jelly like substances that fill the cells
Other organelles “float” in it
Present in both prokaryotic and eukaryotic cells
Prokaryotes
No nucleus
No membrane-bound cells
Simple
DNA is “free floating”
Smaller than eukaryotic cells
ex: bacteria
Eukaryotic Cells
Eukaryotic cells have a membrane-bound nucleus
More complex than prokaryotes
Bigger than prokaryotes
ex: animal cells, human cells, plant cells
Cell Concepts
Membranes are fluid and flexible
Membranes can self-repair
Eukaryotic cells feature membrane bound organelles
Membrane proteins perform special functions
Plant Cell
Cell Wall
Cytoskeleton
Golgi Apparatus
Vacuole
Nucleus
Endoplasmic Reticulum
Ribosome
Chloroplast
Cell membrane
Mitochondria
Cytoplasm
ccccgevnmr
Animal Cell
Cell Membrane
Cytoplasm
Lysosomes
Mitochondria
Endoplasmic Reticulum
Ribosomes
Golgi Apparatus
Nucleus
cclmergn
Vocabulary
Cholesterol: A hydrophobic lipid molecule that changes the fluidity of the membrane
Phospholipid: Lipids with hydrophilic heads and hydrophobic tails that form two layers in the membrane and can move
Transport Proteins: Proteins that help carry substances across the membrane or allow molecules to pass through a channel
Glycolipid: Lipids with carbohydrate chains that serve as cell recognition, helps with cell communication
Glycoprotein: Proteins with carbohydrate chains that serve as cell recognition, helps with cell communication
Protein Channels: Provides safe passage for molecules (ions) that can’t go through the phospholipid bilayer
Cytoskeleton Filaments: Long protein chains that help the cell hold its shape, organelles and other large molecules can travel along these chains like super highways in the cell
Phospholipid Head: Hydrophilic, polar
Phospholipid Tail: Hydrophobic, non-polar
Cell Membrane
Passive Transport (Require NO energy)
Diffusion
When particles flow from high concentration to low concentration
Across cell membranes
Non polar molecules
ex: The smell of hand lotion
Facilitated Diffusion
Particles move from up to down through protein channels
ex: VIP line
Osmosis
Diffusion of water through a semipermeable membrane
Moves through aquaporins
Water moves from an area of high concentration to low concentration
Goal is to balance things out on both sides of the barrier
Active Transport (Requires ATP)
Requires energy
Moves from low to high concentration
Uses protein pumps; pumps change shape to fit particles
Exocytosis
Process used by cells to move substances out of the cell
Vesicles: Contain the substances that are being moved
Endocytosis
Process where the cell takes in materials from the outside environment
Protein Pumps
Help move molecules across the membrane across the gradient
Cell Theory
All living organisms are made from cells
Cell are the basic unit of life
All cells come from other cells
Hypotonic
When comparing two solutions, the solution with the lower amount of solute
Cells swell, then lead to potential bursting
Hypertonic
When comparing two solutions, the solution with the higher amount of solute is called hypertonic
Cells shrink
Isotonic
A solution that has the same concentration of solutes as another solution, leading to no movement of water
Cytolysis
A process that occurs when a cell swells and bursts due to too much water in a hypotonic solution
Can be prevented by the cell wall (in plant cells) by providing structural support
Plasmolysis
A process that occurs when a plant cell loses water and shrinks away from its cell wall due to being placed in a hypertonic solution
Photosynthesis
Chlorophyll and Contrasts
Sunlight is “white” light- actually a mixture of different wavelengths
Photosynthetic organisms capture light energy from sunlight with pigments
Light energy from the sun must be captured for photosynthesis
Pigments: light-absorbing compounds
Chloroplasts: Organelle where photosynthesis takes place
Chloroplast Structure
Chloroplast is stored in the thylakoid membranes

Electron Carriers
A compound that can accept a pair of high energy electrons and transfer them, a long with most of their energy.
A compound called NAPD+ acts as an electron carrier by accepting 2 high energy electrons and 1 hydrogen ion
NADPH can carry the high-energy electrons that were produced by light absorption in the chlorophyll to chemical reactions elsewhere in the cell
Overview of Photosynthesis
Photosynthesis uses the energy of sunlight to convert water and carbon dioxide (low energy reactants) into high energy sugars and oxygen (products)
Chemical Equation: 6CO2 + 6H20 → C6H120O6 + 6O2
Autotrophs do photosynthesis
Photosynthesis and Light
Light-dependent reactions
Light-independent reactions

 oxygen comes from the water,
oxygen comes from the water,
Light Dependent Reactions
Occur in the thylakoid membranes of chloroplasts
Inputs: H20, Light, ADP, NADP+
Outputs: O2, ATP, NADPH
Steps
The sun strikes an electron that is inside PSII. The electron then gets excited
The electron has too much energy so it can’t stay in PSII, so it goes through the electron transport chain. As the electron goes through the ETC, hydrogen and oxygen atoms are going to be released into the thylakoid
The hydrogen that were released during the ETC, are going to be pushed down through ATP synthase and into the stroma. The hydrogen ions that are in the stroma now, turn the ADP into ATP
Eventually, sunlight will excite the electron that is now in PSI and goes through the second ETC. That electron then converts NADP+ into NADPH

Light Independent (Calvin Cycle) Reactions
Occurs in the stroma of the chloroplast
Inputs: CO2, ATP, NADPH
Outputs: Glucose (C6H12O6), ADP, NADP+
Cellular Respiration
Overview of Cellular Respiration
Cellular respiration is a process of energy conversion that releases energy from food in the presence of oxygen
Everything

Cellular Respiration Chemical Equation
In symbols:
6O2 + C6H12O6 → 6CO2 + 6H20 + ATP
ETC Glycolysis Krebs ETC (Majority come from ETC)
In Words:
Oxygen + Glucose → Carbon dioxide + Water + Energy
Glycolysis
Where does it occur?: Cytoplasm
Inputs: ATP, 1 Glucose, NAD+, ADP
Outputs: 2 pyruvic acid, 4 ATP (2 net)
Where do the outputs go?: The pyruvic acid goes to Krebs Cycle, ATP is used by the cell, NADH goes to ETC
Doesn’t require oxygen (anaerobic), quick energy
Krebs Cycle
Where does it occur?: Mitochondrial matrix
Inputs: 2 pyruvic acid, NAD+, FAD, ADP
Outputs: 6 CO2, 8 NADH, 2 ATP, 2 FADH2
Where do the outputs go?: NADH and FADH2 got to ETC, ATP gets used by the cell, CO2 diffuses out and you exhale
Electron Transport Chain
Where does it occur?: Inner Membrane of the Mitochondria
Inputs: NADH, FADH2, O2, ADP
Outputs: 6H2O, 34 ATP, NAD+, FAD
Where do the outputs go?: H2O is used by cells and leaves when exhaled, 34 ATP are used by the cell
Produces a lot of ATP used by the body
Fermentation
In the absence of oxygen, fermentation releases energy from food molecules by producing ATP
Glycolysis must occur first
Lactic Acid Fermentation Equation: Pyruvic Acid + NADH → Lactic Acid + NAD+
Alcoholic Fermentation Equation: Pyruvic Acid + NADH → Alcohol + CO2 + NAD+
Steps of Cellular Respiration
In Glycolysis, glucose molecules are split into two pyruvates
In the Krebs Cycle, the pyruvate molecules from glycolysis go to the mitochondrial matrix to find Coenzyme A. In the presence of NAD+, pyruvate gets attached to Coenzyme A nad is turned into acetyl-CoA
In the Electron Transport Chain, electrons flow through the electron transport chain, causing protons to be pumped from the matrix to the intermembrane space
Comparing Photosynthesis and Cellular Respiration
Photosynthesis “deposits” energy
Cellular Respiration “withdraws” energy
The equations for photosynthesis and cellular respiration are the reverse of each other
The products of one are the reactants of the other
Photosynthesis removes carbon dioxide from the atmosphere, and cellular respiration puts it back
Photosynthesis releases oxygen into the atmosphere, and cellular respiration uses that oxygen to release energy from food
Cell Cycle
Prokaryotic Cells
Most prokaryotes contain a single circular DNA chromosome

Eukaryotic Chromosomes
Eukaryotic cells have much more DNA than prokaryotes have and contain multiple chromosomes.
Complex DNA and protein is referred to as chromatin
Mitosis Phases
Prophase
Metaphase
Anaphase
Telophase
Cytokinesis
Cell Cycle
During the cell cycle, a cell grows, prepares for division, and then divides to form daughter cells. Each daughter cell then moves into a new cell cycle of activity, growth, and division.

Eukaryotic Cell Cycle
There are four stages: G1, S, G2, and M
In eukaryotes, cell division occurs in two main stages. The first stage of the process (the division of the nucleus) is called mitosis. The second stage (the division of the cytoplasm) is called cytokinesis
Interphase Phases
G1 (Cell Growth)
Cells do their most growing
Cells increase in size and synthesize new proteins and organelles
The G stands for “gap”
S (DNA Replication)
Follows G1
New DNA is synthesized as the chromosomes are replicated
By the end, cells contain twice as much DNA as it did at the beginning of the phase
S stands for “synthesis”
G2 (Preparing for Cell Division)
The shortest phase
Many of the organelles and molecules required for cell division are produced
Occurs after DNA replication is completed
M Phase (Cell Division)
Produces 2 daughter cells
It includes mitosis and cytokinesis
Follows interphase
Mitosis
Interphase
Period of the cell cycle between cell divisions in which the cell grows
Divided into 4 stages: G1, S, G2, and M phase
Prophase
The longest phase and may take up to half ot the total time required to complete mitosis
During prophase, the genetic material inside the nucleus condenses and the duplicated chromosomes become visible
Outside the nucleus, a spindle starts to form
A spindle helps separate the duplicated chromosomes
Each duplicated chromosome condenses to appear as two thick strands known as sister chromatids attached at a centromere

Metaphase
The shortest phase
During metaphase, the centromeres of the duplicated chromosomes line up across the center of the cell
Spindle fibers connect the centromere of each chromosome to the two poles of the spindle
At the end, the cell is ready to separate the sister chromatids

Anaphase
Begins when sister chromatids suddenly separate and begin to move apart
During anaphase, the chromosomes separate and move along spindle fibers to opposite ends of the cell.
When anaphase begins, each sister chromatid turns into an individual chromosome

Telophase
During telophase, the chromosomes, which were distinct and condensed begin to spread out into a tangle of chromatin
A nuclear envelope reforms around each cluster of chromosomes, and gradually a nucleolus becomes visible in each daughter nucleus
Mitosis is completed, but the process of cell division has one more step

Cytokinesis
Cytokinesis is the division of the cytoplasm to form two separate cells
Cytokinesis completes the process of cell division by dividing one cell into two
Cytokinesis in Animal Cells
For most animal cells, the cell membrane is drawn inward until the cytoplasm is pinched into two nearly equal parts
Cytokinesis in Plant Cells
A structure known as the cell plate forms halfway between the divided nuclei.
The cell plate gradually develops into cell membranes that separate the two daughter cells.
Regulating the Cell Cycle
Cancer results in uncontrolled cell growth and division.
Rapid growing cancer cells can be targeted by radiation or chemotherapy or by surgically removing the tumor
Mitosis Visual Summary

DNA & Genetics
DNA
There are 4 Nitrogen Bases of DNA:
Adenine
Thymine
Guanine
Cytosine
The bases are connected through hydrogen bonds
Structure:

Punnett Squares
Punnett squares use mathematical probability to help predict the genotype and phenotype combinations in genetic crosses

Summary of Mendel’s Principles
Mendel’s principles of heredity, observed through patterns of inheritance, form the basis of modern genetics
Mendel’s basic principles of inheritance can be used to study the inheritance of human traits and genetic disorders such as cystic fibrosis
2 alleles/gene
Alleles segregate independently during the formation of sex cells *gametes) and are passed on to an offspring independently
Mendel’s Principles of Heredity
The inheritance of biological characteristics is determined by individual units called genes, which are passed from parents to offspring
Where two or more forms (alleles) of the gene for a single trait exist, some alleles may be dominant and others may be recessive
In most sexually reproducing organisms, each adult has two copies of each gene- one from each parent. These genes segregate from each other when gametes are formed
Alleles for different genes usually segregate independently of each other

Other Patterns of Inheritance
Some alleles are neither dominant nor recessive
Incomplete dominance: One allele is completely dominant over another (there is a mix)
Ex: The flower is mixed

Codominance: The phenotypes for both alleles are clearly expressed
Ex: Chicken feather color, human protein controlling blood cholesterol levels

Multiple Alleles: One gene has more than two alleles
Ex: Human blood, fur types, fur color in rabbits

Polygenic Traits: Many traits are produced by the interaction of several genes
Ex: Eye color in fruit flies, coat color in dogs
Trais typically show a wide variety of phenotypes
Based on incomplete dominance
Are often influenced by the environment

Evolution
Who is Darwin?
Father of evolution developed theory of natural selection
Evolution: A change in allele frequencies within a population over time
Natural Selection: Process by which organisms are most suited to their environment survive and reproduce successfully
Things to Know How to Draw
Chloroplasts
Mitochondria
Prophase, Metaphase, anaphase, and telophase
DNA formatting
 Knowt
Knowt
