
1.Esterification
Alcohols and phenols react with carboxylic acids, acid chlorides and acid anhydrides to form esters.
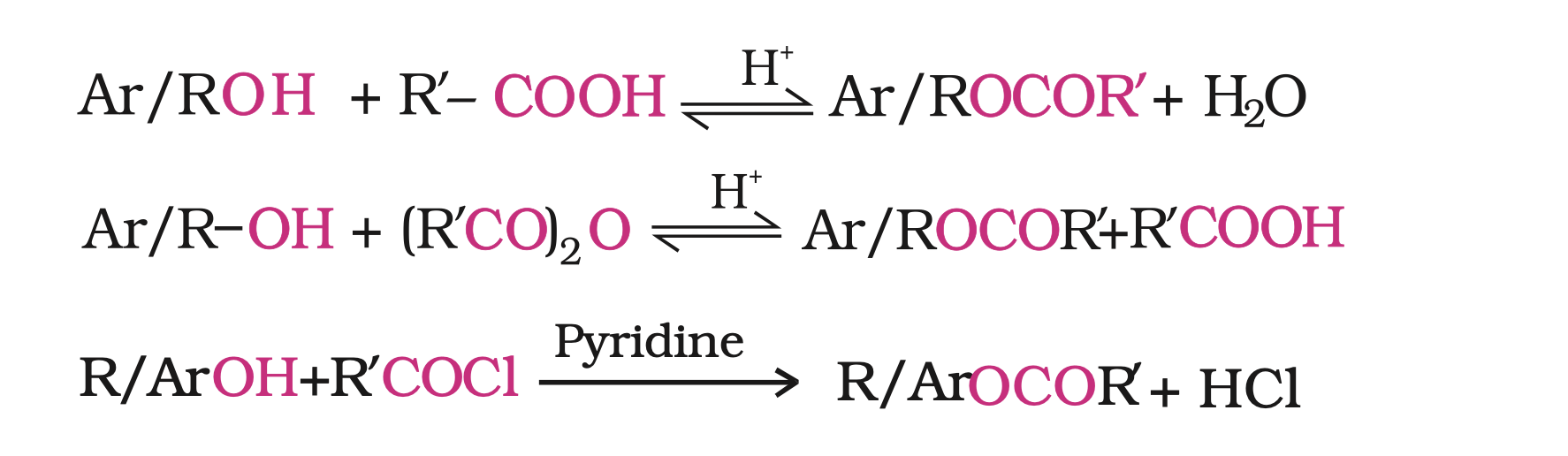
The reaction with carboxylic acid and acid anhydride is carried out in the presence of a small amount of concentrated sulphuric acid.
The reaction is reversible, and therefore, water is removed as soon as it is formed.
The reaction with acid chloride is carried out in the presence of a base (pyridine) so as to neutralise HCl which is formed during the reaction.
It shifts the equilibrium to the right hand side.
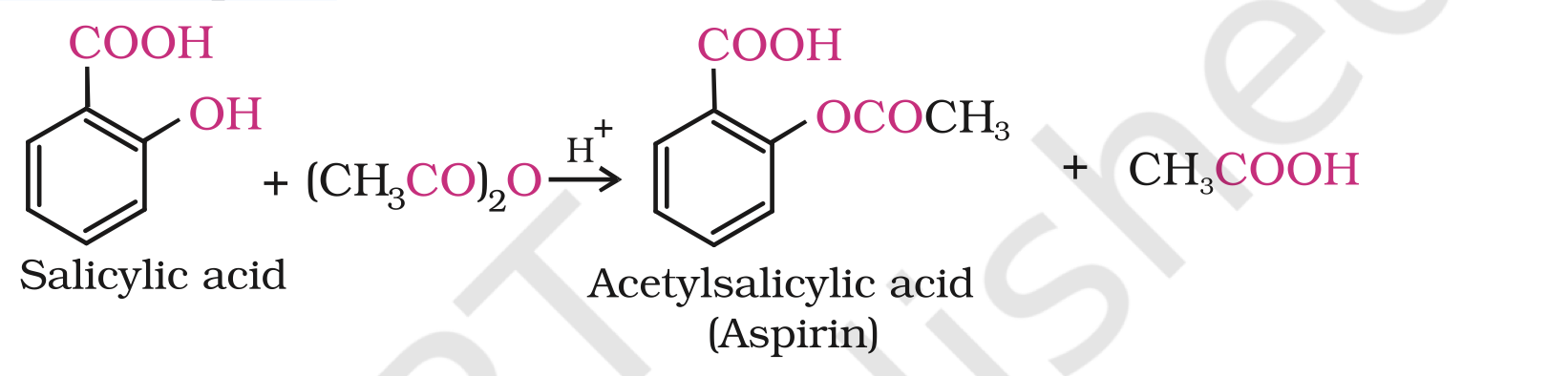
The introduction of acetyl (CH3CO) group in alcohols or phenols is known as acetylation. Acetylation of salicylic acid produces aspirin.
 (b) Reactions involving cleavage of carbon – oxygen (C–O) bond in alcohols
(b) Reactions involving cleavage of carbon – oxygen (C–O) bond in alcohols
The reactions involving cleavage of C–O bond take place only in alcohols. Phenols show this type of reaction only with zinc.
1. Reaction with hydrogen halides: Alcohols react with hydrogen halides to form alkyl halides
 The difference in reactivity of three classes of alcohols with HCl distinguishes them from one another (Lucas test).
The difference in reactivity of three classes of alcohols with HCl distinguishes them from one another (Lucas test).
Alcohols are soluble in Lucas reagent (conc. HCl and ZnCl2) while their halides are immiscible and produce turbidity in solution.
In case of tertiary alcohols, turbidity is produced immediately as they form the halides easily
Primary alcohols do not produce turbidity at room temperature.
2. Reaction with phosphorus trihalides: Alcohols are converted to alkyl bromides by reaction with phosphorus tribromide
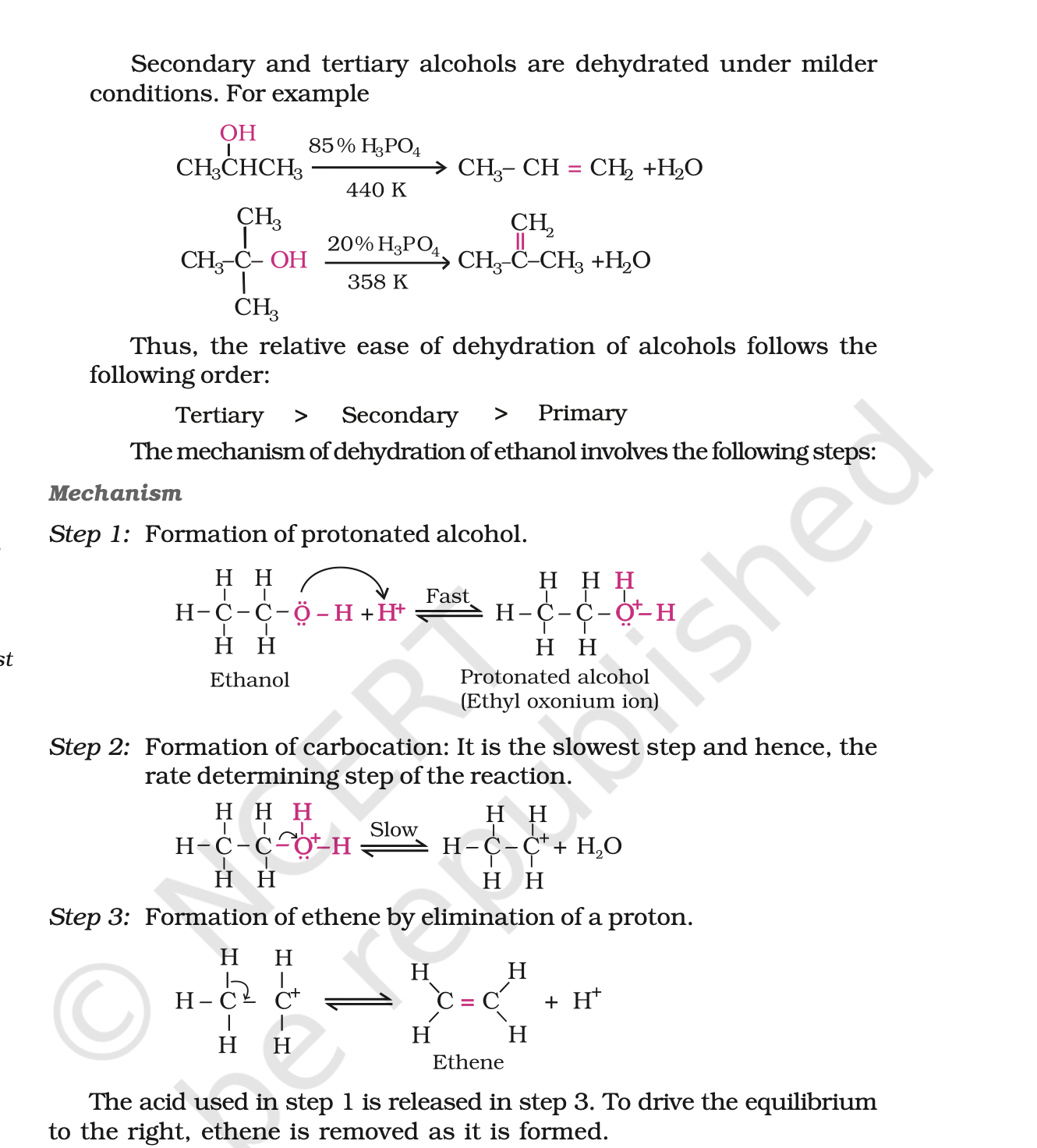
3. Dehydration: Alcohols undergo dehydration (removal of a molecule of water) to form alkenes on treating with a protic acid e.g., concentrated H2SO4 or H3PO4, or catalysts such as anhydrous zinc chloride or alumina.
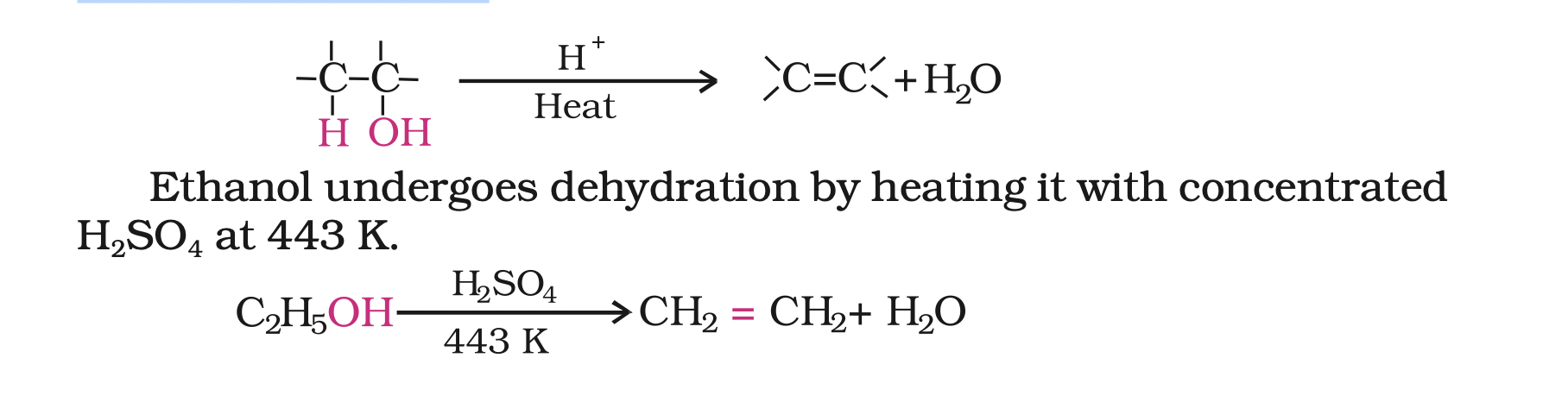
4. Oxidation: Oxidation of alcohols involves the formation of a carbon- oxygen double bond with cleavage of an O-H and C-H bonds.
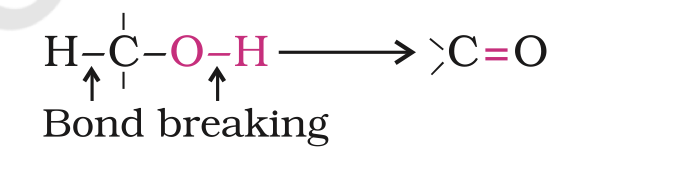
Such a cleavage and formation of bonds occur in oxidation reactions.
These are also known as dehydrogenation reactions as these involve loss of dihydrogen from an alcohol molecule.
Depending on the oxidising agent used, a primary alcohol is oxidised to an aldehyde which in turn is oxidised to a carboxylic acid.
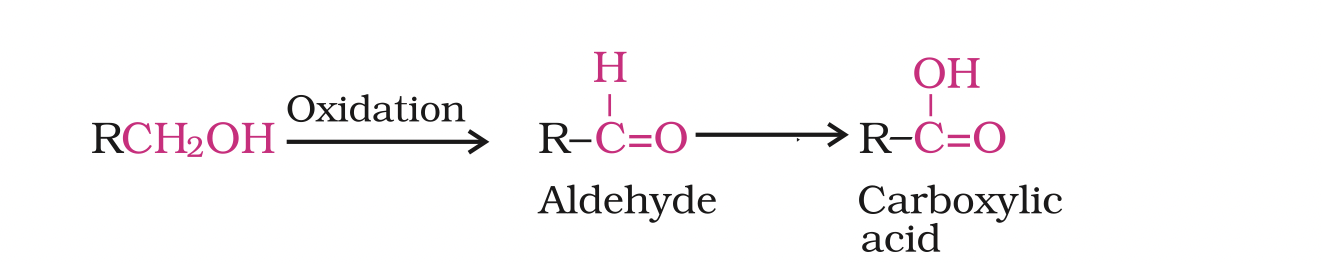
Strong oxidising agents such as acidified potassium permanganate are used for getting carboxylic acids from alcohols directly.
CrO3 in anhydrous medium is used as the oxidising agent for the isolation of aldehydes.
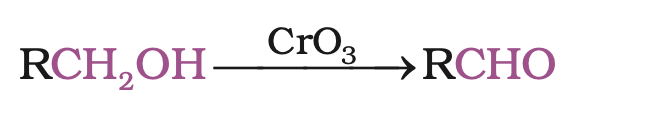 A better reagent for oxidation of primary alcohols to aldehydes in good yield is pyridinium chlorochromate (PCC), a complex of chromium trioxide with pyridine and HCl
A better reagent for oxidation of primary alcohols to aldehydes in good yield is pyridinium chlorochromate (PCC), a complex of chromium trioxide with pyridine and HCl
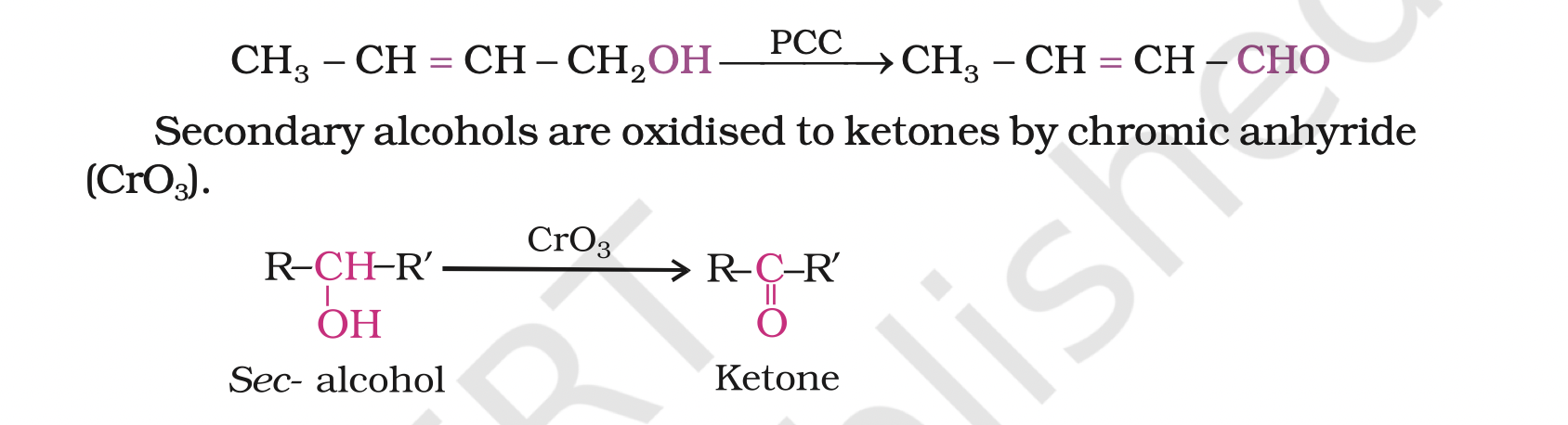
Tertiary alcohols do not undergo oxidation reaction.
Under strong reaction conditions such as strong oxidising agents (KMnO4) and elevated temperatures, cleavage of various C-C bonds takes place and a mixture of carboxylic acids containing lesser number of carbon atoms is formed.
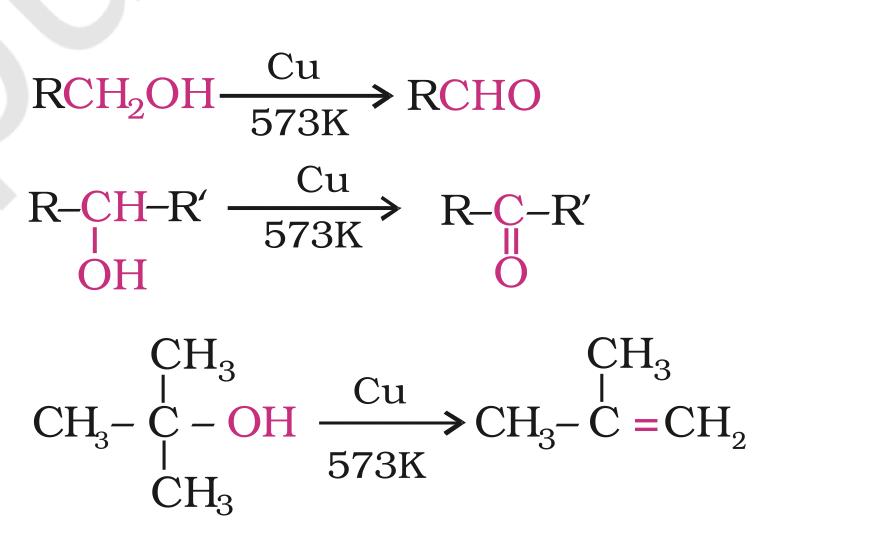
When the vapours of a primary or a secondary alcohol are passed over heated copper at 573 K, dehydrogenation takes place and an aldehyde or a ketone is formed
tertiary alcohols undergo dehydration
1.Esterification
Alcohols and phenols react with carboxylic acids, acid chlorides and acid anhydrides to form esters.
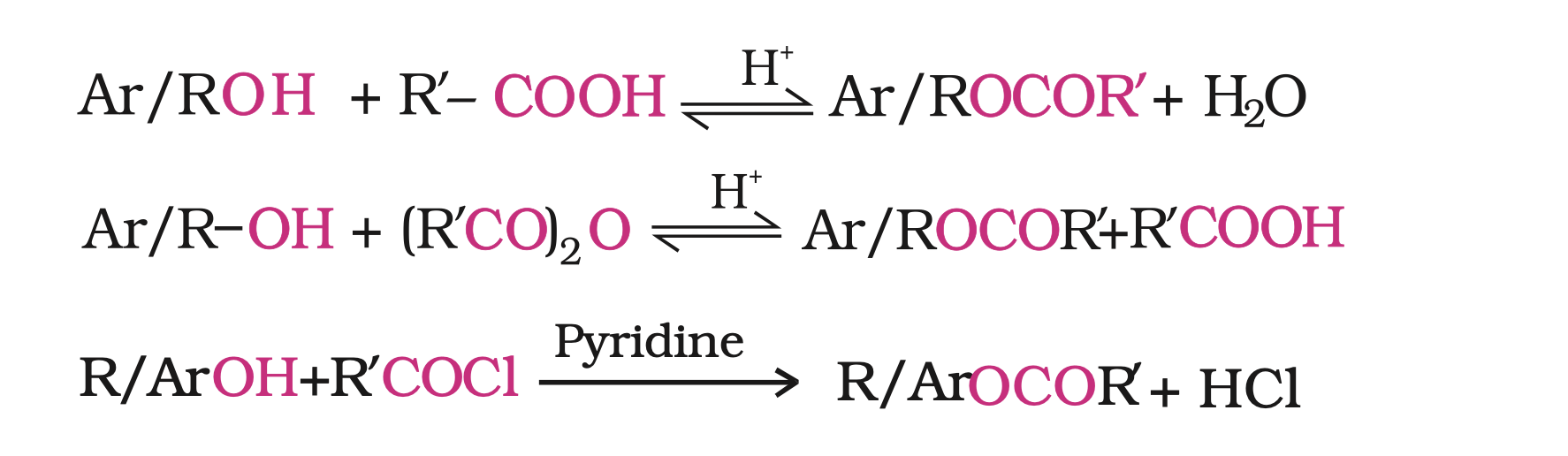
The reaction with carboxylic acid and acid anhydride is carried out in the presence of a small amount of concentrated sulphuric acid.
The reaction is reversible, and therefore, water is removed as soon as it is formed.
The reaction with acid chloride is carried out in the presence of a base (pyridine) so as to neutralise HCl which is formed during the reaction.
It shifts the equilibrium to the right hand side.
The introduction of acetyl (CH3CO) group in alcohols or phenols is known as acetylation. Acetylation of salicylic acid produces aspirin.
 (b) Reactions involving cleavage of carbon – oxygen (C–O) bond in alcohols
(b) Reactions involving cleavage of carbon – oxygen (C–O) bond in alcohols
The reactions involving cleavage of C–O bond take place only in alcohols. Phenols show this type of reaction only with zinc.
1. Reaction with hydrogen halides: Alcohols react with hydrogen halides to form alkyl halides
 The difference in reactivity of three classes of alcohols with HCl distinguishes them from one another (Lucas test).
The difference in reactivity of three classes of alcohols with HCl distinguishes them from one another (Lucas test).
Alcohols are soluble in Lucas reagent (conc. HCl and ZnCl2) while their halides are immiscible and produce turbidity in solution.
In case of tertiary alcohols, turbidity is produced immediately as they form the halides easily
Primary alcohols do not produce turbidity at room temperature.
2. Reaction with phosphorus trihalides: Alcohols are converted to alkyl bromides by reaction with phosphorus tribromide
3. Dehydration: Alcohols undergo dehydration (removal of a molecule of water) to form alkenes on treating with a protic acid e.g., concentrated H2SO4 or H3PO4, or catalysts such as anhydrous zinc chloride or alumina.
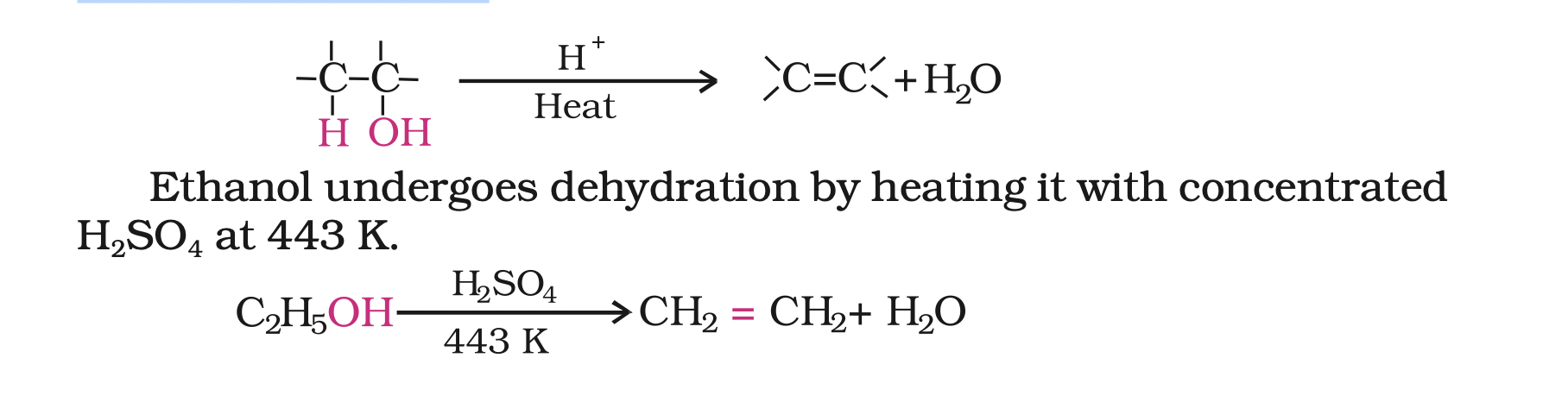

4. Oxidation: Oxidation of alcohols involves the formation of a carbon- oxygen double bond with cleavage of an O-H and C-H bonds.
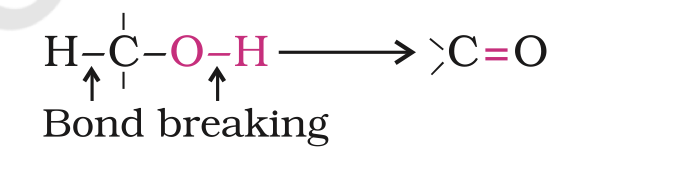
Such a cleavage and formation of bonds occur in oxidation reactions.
These are also known as dehydrogenation reactions as these involve loss of dihydrogen from an alcohol molecule.
Depending on the oxidising agent used, a primary alcohol is oxidised to an aldehyde which in turn is oxidised to a carboxylic acid.
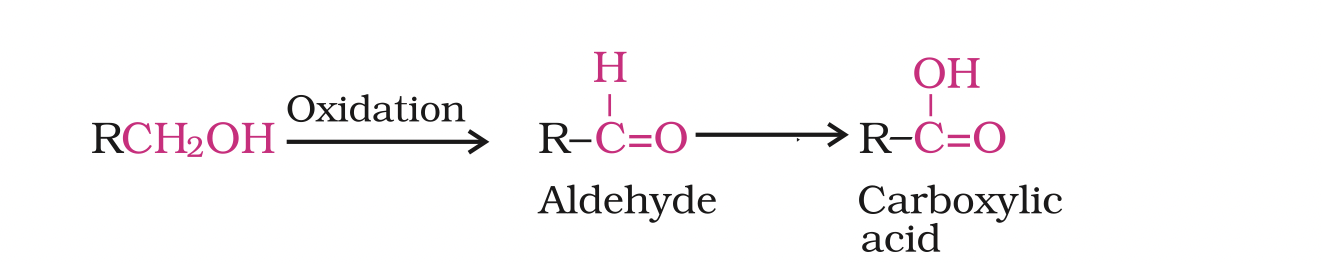
Strong oxidising agents such as acidified potassium permanganate are used for getting carboxylic acids from alcohols directly.
CrO3 in anhydrous medium is used as the oxidising agent for the isolation of aldehydes.
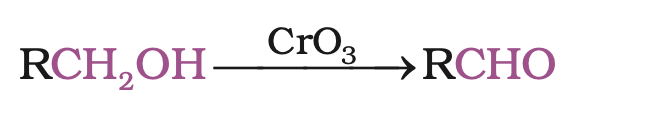 A better reagent for oxidation of primary alcohols to aldehydes in good yield is pyridinium chlorochromate (PCC), a complex of chromium trioxide with pyridine and HCl
A better reagent for oxidation of primary alcohols to aldehydes in good yield is pyridinium chlorochromate (PCC), a complex of chromium trioxide with pyridine and HCl

Tertiary alcohols do not undergo oxidation reaction.
Under strong reaction conditions such as strong oxidising agents (KMnO4) and elevated temperatures, cleavage of various C-C bonds takes place and a mixture of carboxylic acids containing lesser number of carbon atoms is formed.
When the vapours of a primary or a secondary alcohol are passed over heated copper at 573 K, dehydrogenation takes place and an aldehyde or a ketone is formed
tertiary alcohols undergo dehydration

 Knowt
Knowt