Chapter 18 - Cell Division Cycle
Cell division is required for organism growth, tissue renewal, and healing after wounds. Healing from a wound requires a cell signal, which is done via paracrine signaling. They tell the tissue to divide, fill in the wound, close it, and then stop dividing once it is closed. Cells must be pushed into cell division
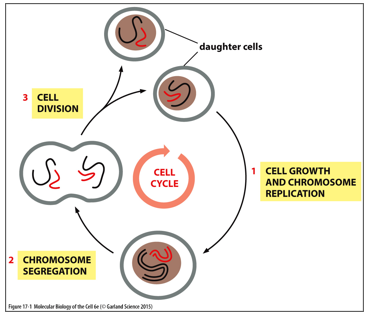
In order for a cell to divide, chromosomes must first be replicated by DNA Polymerase I. Mitosis splits the chromosomes, and cytokinesis splits daughter cells. Mitosis is microtubule dependent, while cytokinesis is actin dependent
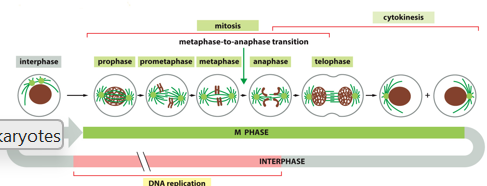
The G1 phase is where the cell typically grows, the S phase is where DNA is replicated. G2 prepares the cell for division, cell division is mitosis and cytokinesis.
Checkpoints help make sure the cell cycle is controlled.
The first G1/S (restriction point) commits a cell to either dividing or entering G0. This is where a cell will decide if it should terminally differentiate (like neural cells)
The second G2/M checks if all DNA is only replicated once, and that none of it is damages
M checkpoint is anaphase promotion, it only occurs is chromosomes are aligned in the middle
The job of stem cells is to divide, meaning they are constantly undergoing the cell cycle and cell division. Most somatic cells typically are in G0, but can be pushed back into the cell cycle to divide or rarely even back into stem cells
Cyclin dependent kinases (CDKs) are what regulates the cell cycle. These kinases rely on cyclin in order to be active, giving them their name. Cyclins prepare the cell and regulate enzymes. There are G1/S-Cdks, S-Cdks, and M-Cdks, all of which are present and active at varying points to the cell cycle. Anaphase can only begin once levels of all cyclins are very low
If after the G1 phase the environment is unfavorable, Cdk inhibitors will block entry into the S phase. At the G2/M checkpoint, if DNA replication isn’t complete of if there is damage to the DNA, inhibition of activating phosphatase (Cdc25) blocks entry of the cell into mitosis. During the M phase, if chromosomes are not properly attached to spindles, inhibition of APC/C activation delays exit from mitotic states
The cell cannot enter anaphase without destructing M-cdks. This is a restriction point, a restriction point is passed once certain levels of certain CDKs is met. They are blocks with certain requirements, if they aren’t met the cell may go into apoptosis (typically)
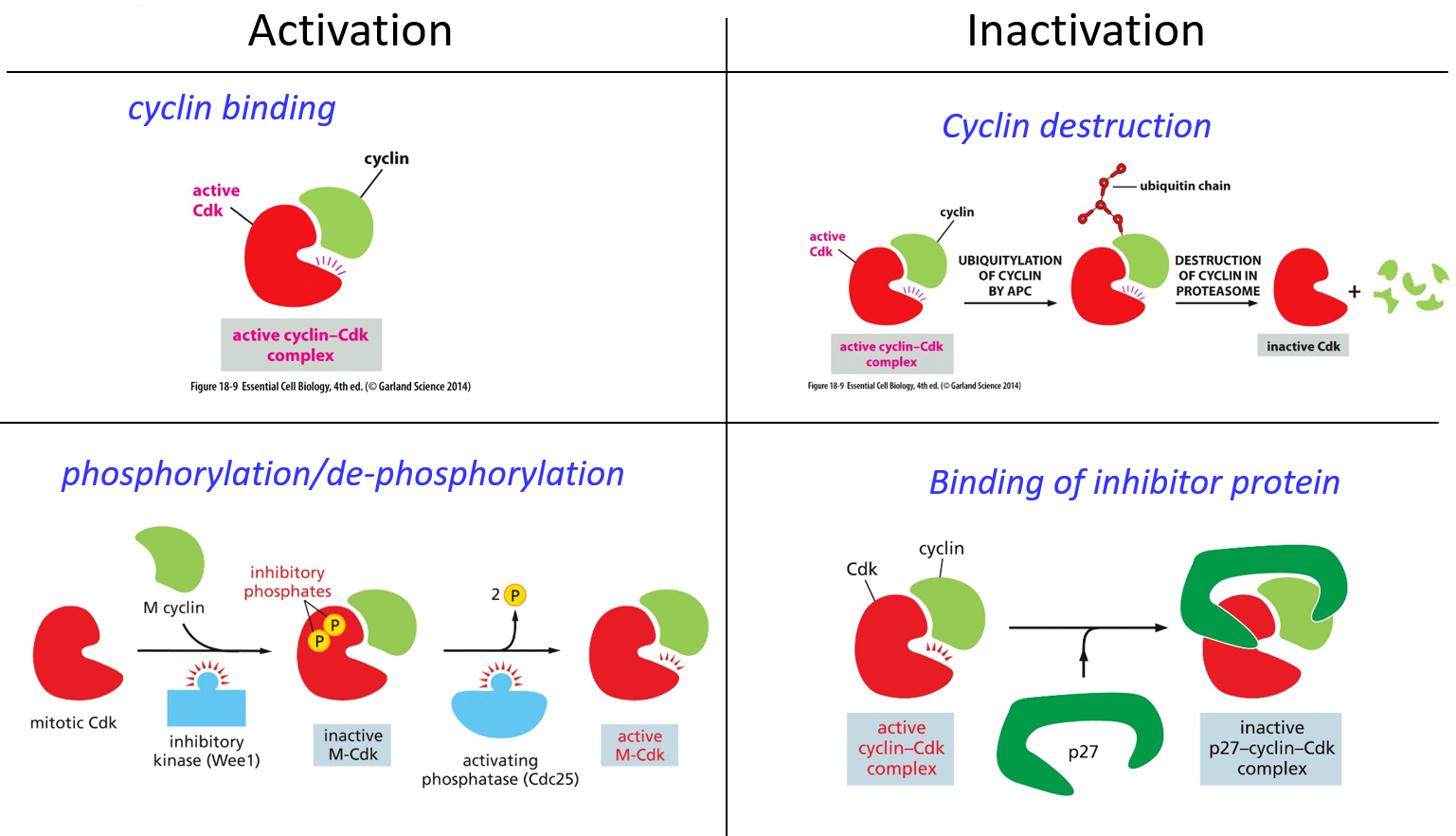
Activation of a cyclin involves the formation of the cyclin-cdk complex. Cyclin binding activates CDKs It also uses phosphorylation/dephosphorylation. A given CDK will be bound by a cyclin, but a kinase (Wee1) will phosphorylate it twice, inactivating the CDK until a phosphatase takes away the two inhibitory phosphates. For inactivation, cyclin deconstruction will use ubiquitylation. An inhibitor protein like p27 may also bind to the active cyclin-cdk complex, making the whole complex inactive.
DNA damage may stall a cell in G1, damage may activate protein kinases that phosphorylate p53. Without damage, p53 is degraded in proteasomes but while active and phosphorylated, it will bind to the regulatory site of the p21 gene, and create p21, a cdk inhibitor protein. p21 will bind to cyclin-cdk complexes, and inactivate the cyclins from pushing the cell past the G1 phase. Expression of the inhibitor protein will halt the cell cycle, allowing it to repair DNA damage before continuing on
The M phase involves mitosis and cytokinesis. This is conserved in eukaryotes, and it the segregation of DNA that helps maintain proper numbers of chromosomes. The 5 dynamic sequences of events result in genetically identical daughter cells
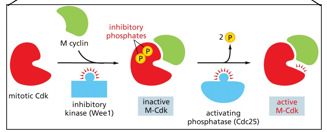
Mitosis is initiated by the m cyclin, which is activated by mitotic cdk with the help of inhibitory kinase (Wee1)
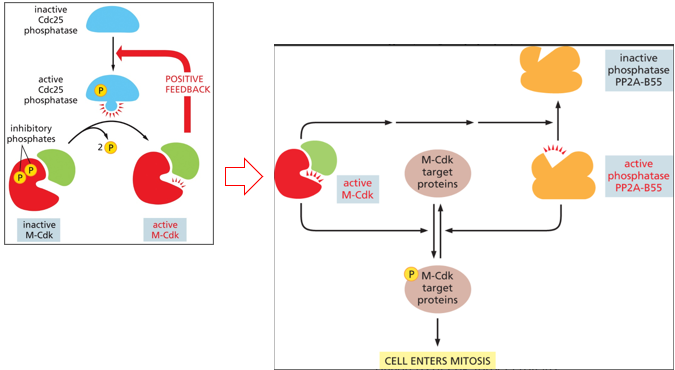
There is also positive and negative feedback with m-cdk, more M-CDK there is, the more can be activated. It activates a phosphatase that allows for the dephosphorylation of the inhibitory phosphates added by Wee1 to the M-CDK/cyclin complex
During mitosis, protein complexes facilitate condensation of DNA into mitotic chromosomes. Intra-chromatid has many DNA loops around condensation rings, while inter-chromatid forms sister chromatids. Condensins condense DNA strands, once duplicated cohesins will hold sister chromatids together
During mitosis, the microtubules of the cytoskeleton help form mitotic spindles to bring chromosomes to either side. Actin and myosin filaments form the contractile ring, which ultimately forms cleavage and separates daughter cells
The centrosome will duplicate and form poles for mitotic spindles
During prophase, the nuclear envelope is still intact, and the centrosome starts to form mitotic spindles. Chromosomes (each with 2 sister chromatids) condense. There is aster formation, the separation of centrosomes, movement to opposite ends of the cell, multiplication and elongation of microtubules, and formation of a bipolar mitotic spindle
During prometaphase, the nuclear envelope breaks down, microtubules ‘fish’ for chromosomes via preferential growth from both kinetochores. There is stable association, congression to the center of the mitotic spindle via kinetochore motor proteins, microtubule length adjustment, and alignment at the spindle equator
The kinetochore is a multiprotein complex on the centromere that acts as an adaptor for microtubule attachment to the chromosome. It contains motor proteins for segregation, and it utilized during the mitotic checkpoint. The only stable configuration of the kinetochore is each sister chromatid being attached to by opposite centrosomes, not one centrosome for both or both centrosomes to one
During metaphase, chromosomes are aligned at the equator of spindles, halfway between poles. Kinetochore microtubules on each sister chromatid attach to opposite poles of the spindle
During anaphase, sister chromatids are separated synchronously and pulled towards separate spindle poles. Microtubules get shorter and pull apart, contributing to chromosome segregation
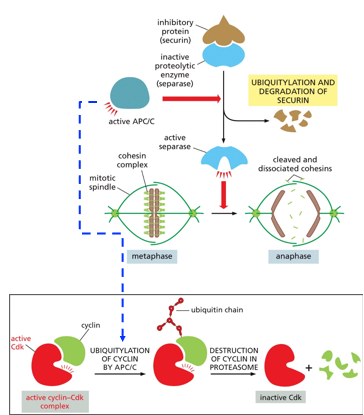
The Anaphase Promoting Complex (APC/C) signals the cell into anaphase. A complex of securin and separase will allow for the degradation of cohesins, breaking down the centromere. Securin deactivates separase from acting until it is needed. The APC/C will ubiquitylate this complex, allowing the separase to activate. At this time, M-CDKs are also ubiquitylated
Anaphase has 2 parts, Anaphase A where chromosomes move and Anaphase B where spindle poles move. Sister chromatids sever their connections, via the breakdown of cohesins. There is slow movement towards opposite pulls without entanglement, and the shortening of chromosomal microtubules (subunits lost from the + end). In Anaphase B, the dyneins pull chromosomes to move faster and increase the rate of movement.
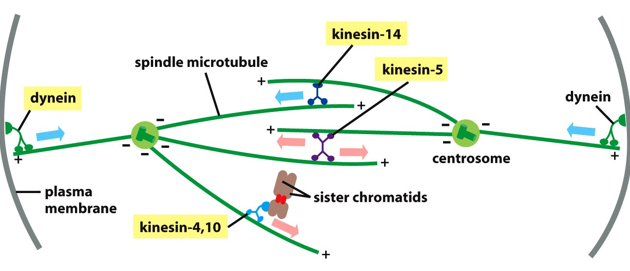
There are various kinesins and dyneins in this process, dyneins connect the centrosome to the plasma membrane, and help move chromosomes toward poles
Telophase is the mitotic exit, when two sets of chromosomes arrive at poles of the spindles. New nuclear envelops form around each set, marking the end of mitosis
For a centromere, the synaptonemal complex is what holds maternal and paternal chromatids together, comprised of cohesins
Cytokinesis occurs after telophase, and for animals cells is the formation of the contractile ring of actin, which pinches the cell into two daughter cells each with one nucleus, splitting cytoplasm in the process
The contractile ring is make of actin and myosin II, they form a cortical ring that lies in the same plane as the equator plane for mitotic spindles
Meiosis is the reduction of the number of chromosomes (diploid → haploid). Meiosis I is for non-identical but homologous chromosomes, of which they pair and genetically recombine and undergo random segregation. Meiosis II consists of the separation of non-identical chromatids, and results in 4 haploid daughter cells
Apoptosis is programmed cell death, and helps maintain homeostasis. The cytoskeleton will collapse, the nuclear membrane will disassemble, DNA will fragment, and apoptotic bodies will form. Membranes will remain intact
Necrosis is different that apoptosis, in necrosis the cell swells, membranes break, the cell lyses eliciting an inflammatory response, DNA fragmentation is random, there is free radical damage and ATP depletion, and affects whole areas of tissue. The cell swells, chromatin lumps, and the membrane bursts releasing cellular components outside, leading to an inflammatory response.
With apoptosis, the cell shrinks, chromatin compacts, the cell fragments into apoptotic bodies (each with the membrane still intact)
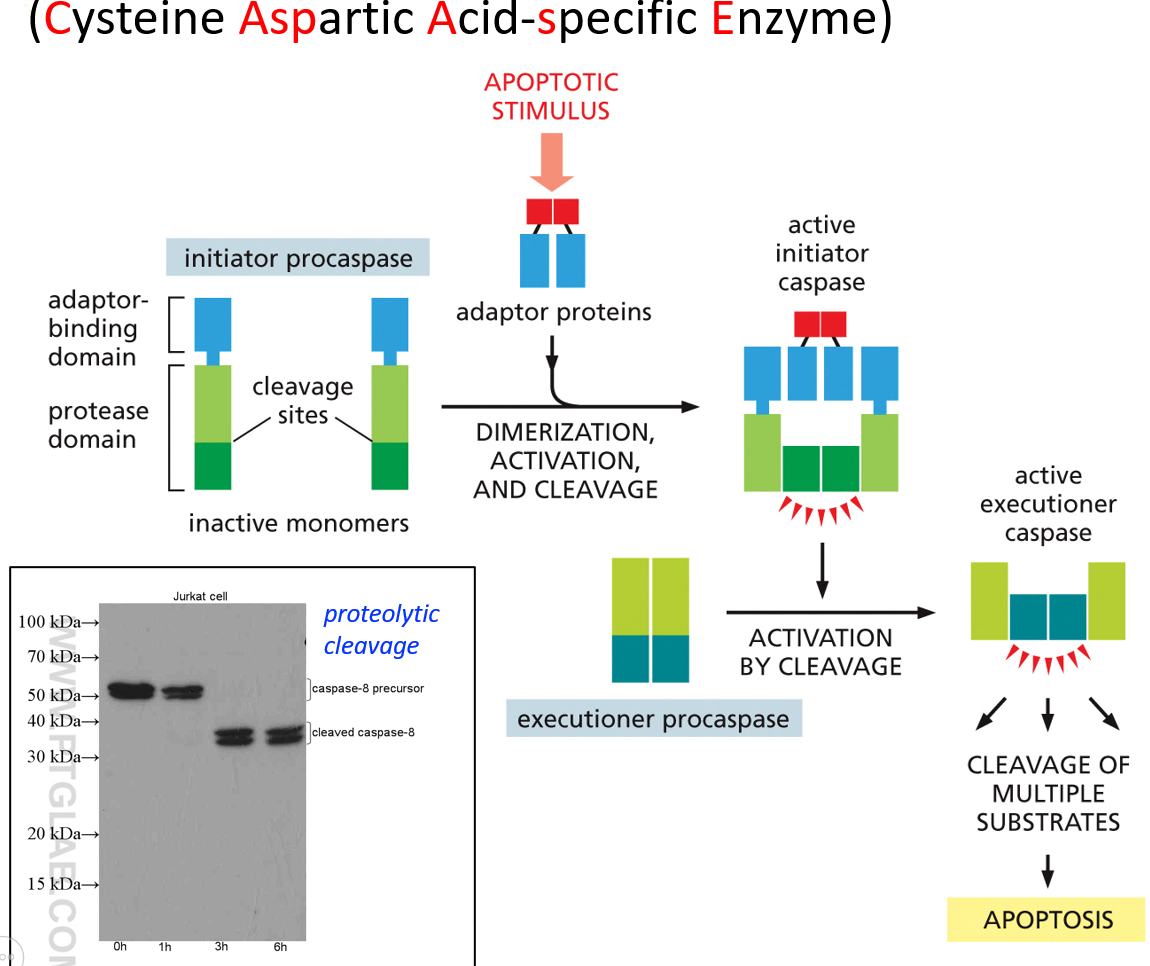
Caspases are what tell the cell to not go into apoptosis. The active site has cysteine. They can either be initiator caspases or executioner caspases. An apoptotic signal will activate the initiator ones, which then activate the executioner ones, which then initiate apoptosis. These are all present in the cytosol and inactive. The initiator cleaves part of the executioner, which activates it.
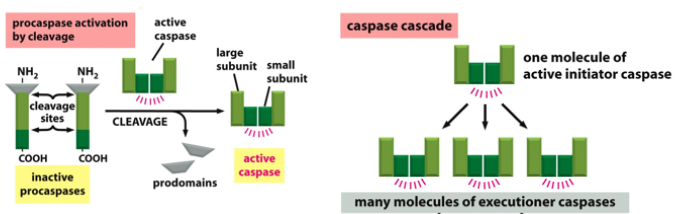
Proteolytic cleavage is the process of activating the executioner. Once the initiator is activated, there is no reversal and apoptosis will occur. There are extrinsic or intrinsic signals, which activate unique initiator caspases, but activate the same executioner caspases. This whole process is a caspase cascade. There are far more executioner than initiator caspases. They are also self activating, leading to positive feedback.
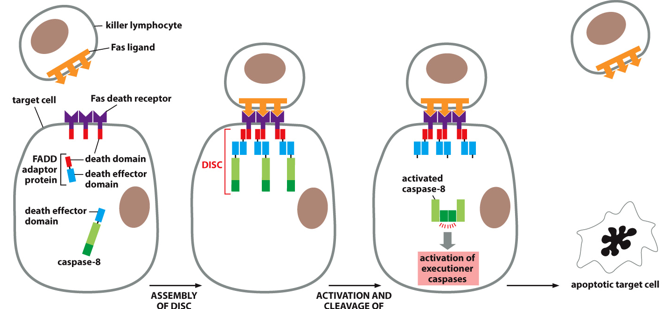
The Fas pathway is an extrinsic trigger of apoptosis via with extracellular signals. A Fas ligand on a killer lymphocyte may bind to Fas death receptors on a target cell. This will start the formation of the death complex (DISC), which will activate initiator caspases (caspase-8 specifically), activating executioner caspases, causing apoptosis in an immune response
There are also intrinsic triggers that induce an intracellular response to things like oxidative stress, DNA damage, or age. This uses cytochrome C (typically part of the ETC), which is released from the mitochondria, where they then activate an adaptor protein. Many of these activated adaptor proteins will assemble into an apoptosome, that with procaspase 9 (an initiator caspase) will grow via caspase cascade leading to apoptosis
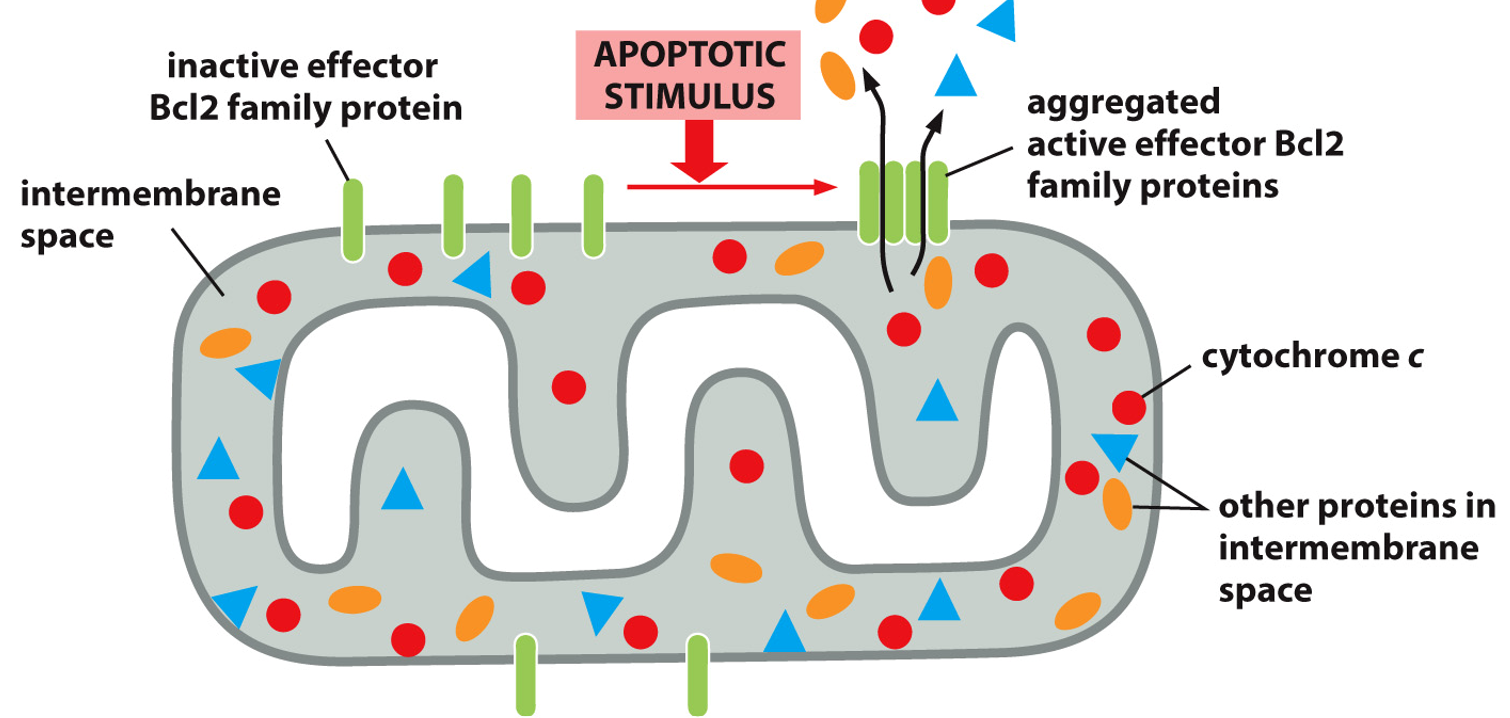
Cytochrome C is in the intermembrane space of the mitochondria. An apoptotic stimulus will cause Bcl2 proteins, previously inactive, to come together to form an aggregate and activate. This will allow cytochrome C out down it’s concentration gradient, and kickstart apoptosis from an intrinsic signal.
Apoptosis will happen without survival factors, when the cell receives these a receptor will activate, and via a pathway activate a transcription regulator, blocking transcription of the Bcl2 gene and thus the protein. A survival signal may activate a transcriptional regulator, increasing transcription and production of a Bcl2 family protein, that will block Bcl2 from forming a channel and starting apoptosis.
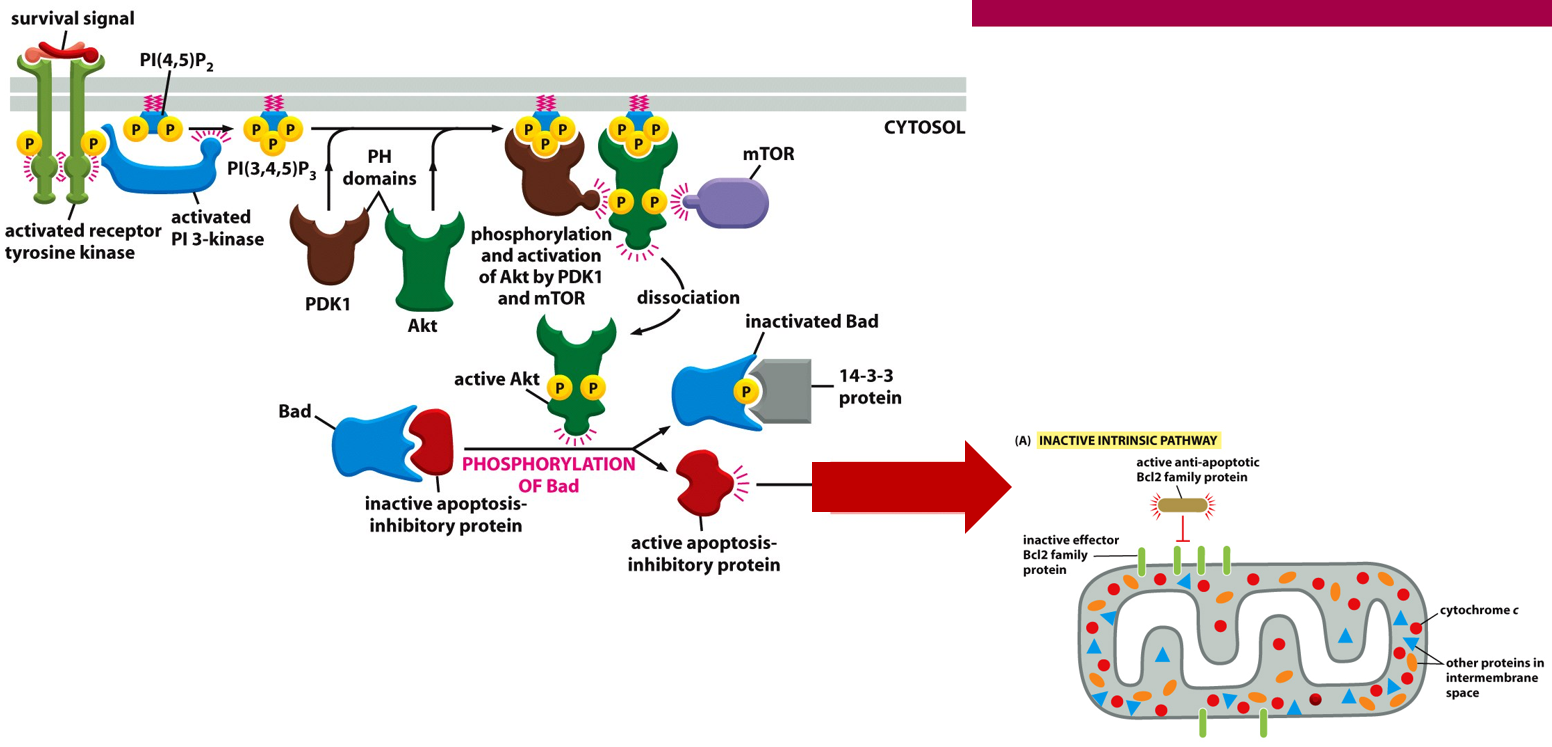
The PI-kinase-Akt pathways also plays a role in cell survival. The receptor signal will come into the Rtk, eventually phosphorylating Akt and inactivating Bad
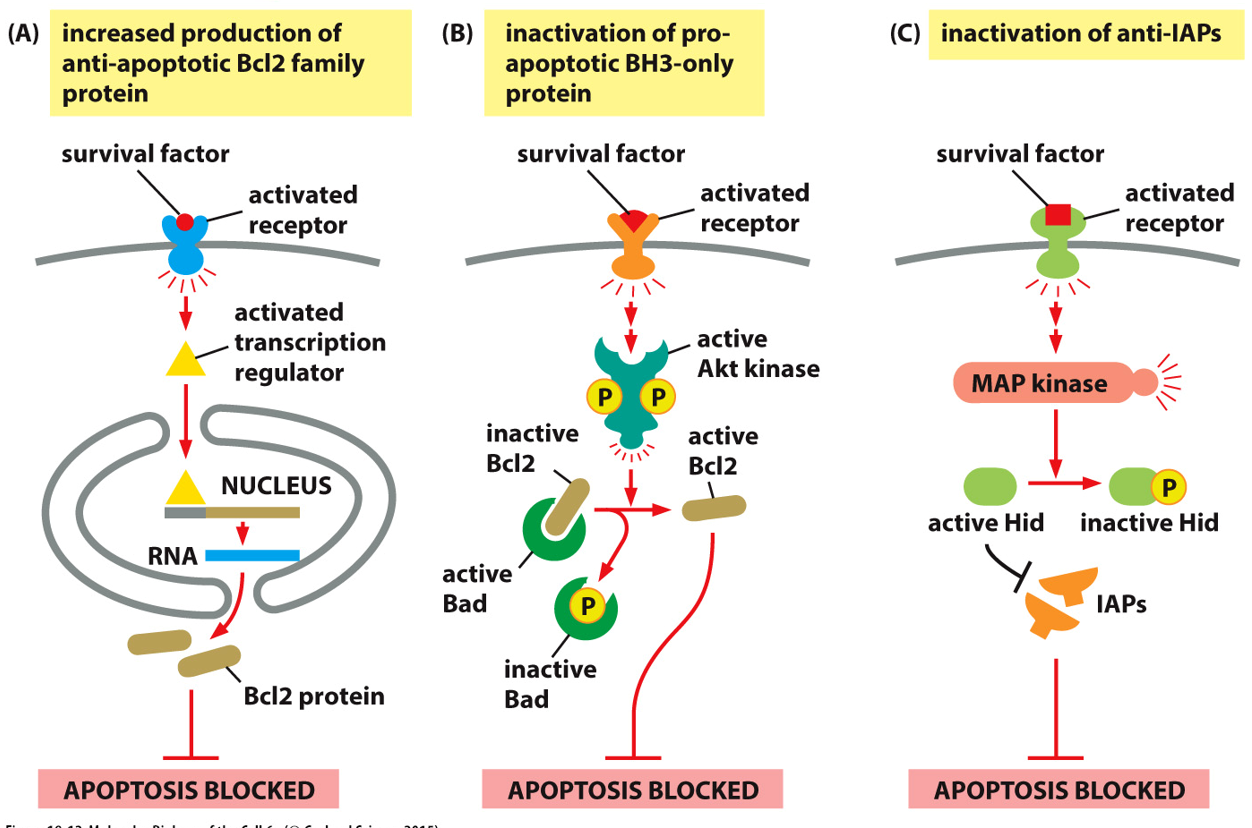
There are many ways to prevent apoptosis, many proteins that stop it are known as inhibitors of apoptosis proteins (IAP). Apoptosis is blocked if there are more Bcl2 regulators, more BH3 regulation, or inactivation of anti-IAPs. These prevent accidental apoptosis/activation of caspases.