Gas laws
Concept of a thermodynamic system
A thermodynamic system is a system where particles are in ceaseless interaction with each other. Example: blood, human body etc.
Types
- Open system: substance, matter and energy is exchanged with the environment. E.g; air reaction with substances in a test tube.
- Closed system: substance and matter are not exiting but energy can move in or out of the system. E.g; a hermetically sealed test tube.
- Isolated system: no matter or energy goes in or out of the system. Complete isolation from environment.
Properties of thermodynamic systems
• Extensive variable: if a system is made of subsystems its change is proportional to each subsystems size. Example; mass, volume, entropy, particle count.
• Intrinsic variables: if a system is made of subsystems it doesn’t change. Example; pressure, temperature.
Properties of ideal gas as a thermodynamic model system
According to ideal gas model gas particles can move freely according to the volume available to them, they usually collide with themselves and the container walls. They move in a straight line but if they experience an elastic collision they can be steered off and accelerate or decelerate.
Gas laws
An isolated system is a system at equilibrium, the variables do that change without external effects.
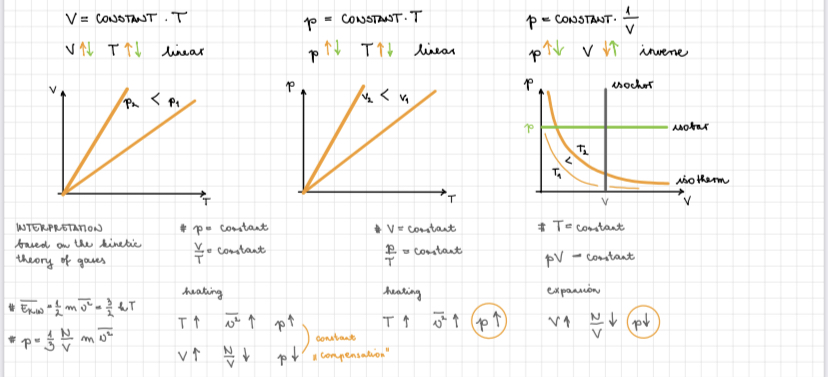
pV/T = constant — General has law
p is pressure, v is volume, T is temperature.
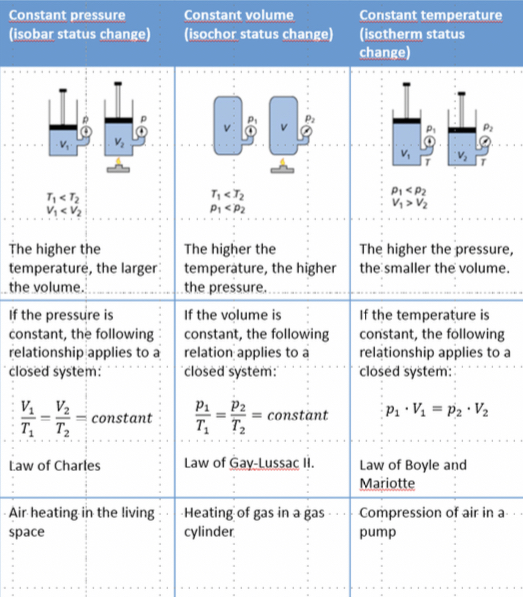
• Boyles law:
At constant temperature the pressure is inversely proportional to the volume.
p1•V1 = p2•V2
• Charles law:
At constant pressure the volume is directly proportional to the temperature.
T1/V1 • T2/V2
• Gay-Lussac’s law:
At constant volume pressure is inversely proportional to the temperature.
p1/T1 • p2/V2
Ideal gas law
The equation of state:
pV = nRT
p is pressure, V is volume, n is number of moles, R is universal gas constant, T is temperature.
n = m/M = N/NA
m is mass, M is molar mass, N is matter density, NA is avogadro’ constant; 6.0 x 10^-23mol.
pV = N/NA x RT = NkT
k = R/NA and represents Boltzmann constant; 1.38 x 10^-23J/K
* standard state of gases;
n= 1mol, p = 101.325kPa, T = 273.15K, V = 22414cm^3.
Phases of water
Graphic representation in which individual states are thermodynamically stable. ==Boiling point is the point where vapour pressure is equal to atmospheric pressure at constant pressure.==
==In a closed vessel temperature, pressure and density of the steam continuously increases, when vapour density is equal to the liquid density it is the critical temperature.==
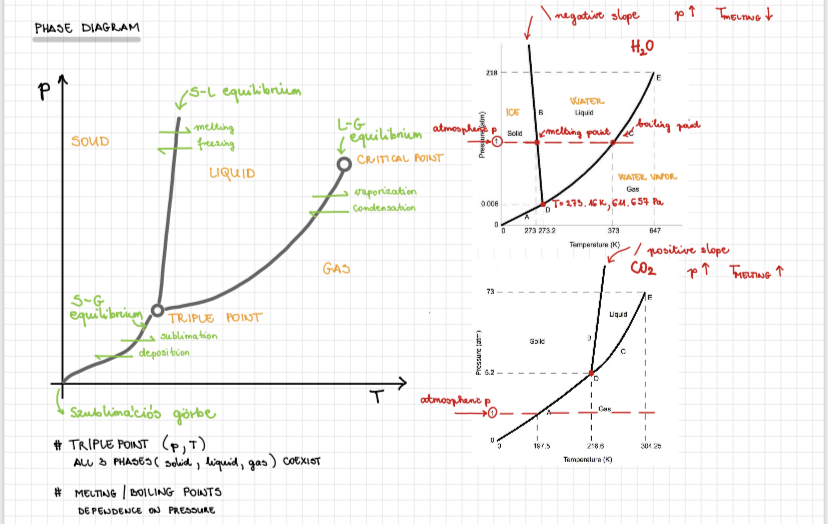
Characteristics of phase diagrams
From:
a) Solid to Liquid - Melting
b) Liquid to solid - Freezing
c) Gas to liquid - Condensation
d) Liquid to Gas - Vaporisation
e) Gas to Solid - Depoaiotion
f) Solid to Gas - Sublimation
Thermal expansion of gases and liquids
Gases: when heated gases expand more than liquids.
Change in V = V• change in T •thermal coefficient
Liquids: increase in temperature equals increase in volume.
Change in V= V•change in T•volume coefficient