2.7 Alcohols
In this topic we will discuss the structure of alcohols, including different methods of producing alcohols both on a molecular, and industrial level.
Making Alcohol:
Hydration of Ethene
C2H4 + H2O ≠ C2H5OH
Ethane → Ethanol = Exothermic
Ethanol → Ethane = Endothermic
This reaction is done through the mechanism of electrophilic addition, where the double bond in ethene is broken and the H2O molecule is added to the carbocations.
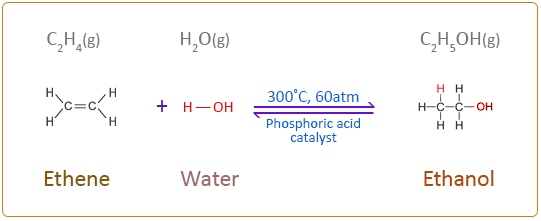
Conditions for this reaction:
300ºC temperatures
60atm
Phosphoric acid catalyst
Fermentation of sugars
Biofuels are made by fermenting sugars from plants with a yeast catalyst. The process requires a warm environment and takes place over a few days. The yeast produces enzymes that break down the sugar, anaerobically, forming a biofuel such as ethanol.
C6H12O6 → 2C2H5OH + 2CO2
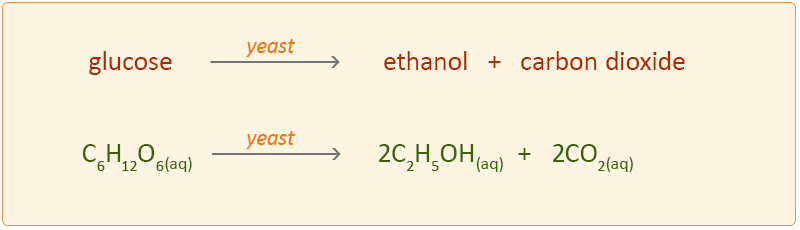
The carbon dioxide escapes as a gas; the ethanol ( boiling point of approx 80) has to be separated from the mixture by fractional distillation.
Biofuels:
Biofuels are fuels that are produced by living organisms. The two main types are bioethanol and biodiesel.
Bioethanol - Obtained from sugars in plants by fermentation.
Biodiesel - Obtained from oils and fats that are present in the seeds of some plants.
Advantages of their use: | Disadvantages of their use: |
Renewable - Fossil fuels will run out, biofuels can be produced from plants and animals | Land use - The use of land for fuel means it isn’t being used for food. Could encourage forest destruction. |
Greenhouse Gasses - Overall carbon dioxide production is less | Use of resources - To grow crops you need large amounts of fertiliser, which could cause water pollution. |
Economic and political security - Countries without fossil fuels are less dependent on changes in the price and availability of imported fossil fuels | Carbon neutrality - Fuel/ resources are needed to build biofuel factories and transport the product, (not very carbon neutral). |
 Knowt
Knowt