3_Water_and_Life_Lecture_Slides
Importance of Water in Biology
Water is essential for life, contributing significantly to various biological processes.
Unique Properties of Water
Polarity and Hydrogen Bonds
Water molecules are polar due to uneven distribution of electrons; the electrons spend more time near the oxygen than the hydrogen.
This polarity enables water to form hydrogen bonds.
Emergent Properties of Water
Water's properties that support life include:
Cohesive behavior: High surface tension and support for organisms.
Ability to moderate temperature: Water can absorb/release heat with minimal temperature change.
Expansion upon freezing: Ice is less dense than liquid water, allowing it to float.
Versatility as a solvent: Water dissolves a wide range of substances.
Cohesion and Adhesion
Cohesion: Hydrogen bonds hold water molecules together, creating high surface tension.
Adhesion: Water's attraction to other substances aids in water transport in plants.
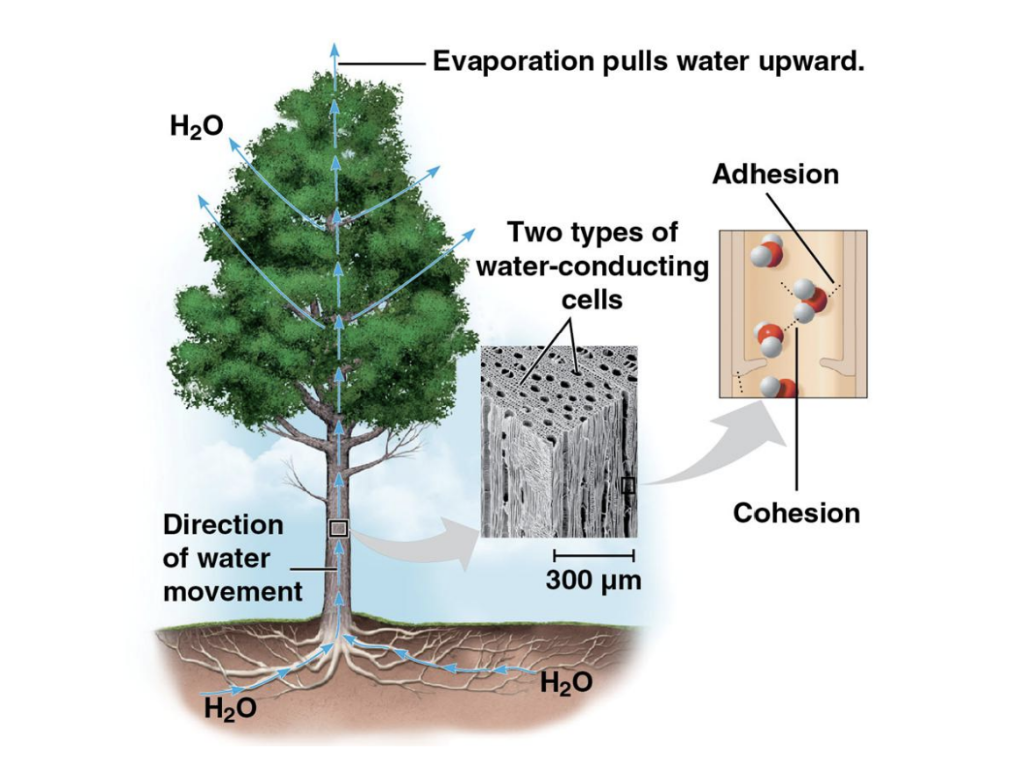
Temperature Regulation
Water absorbs heat, stabilizing temperature changes in organisms and environments.
Specific Heat: Water's high specific heat is due to hydrogen bonding, enabling it to resist temperature changes.
Kinetic energy: the energy of motion.
Thermal energy: the kinetic energy associated with random motion of atoms or molecules.
Heat: thermal energy transfer from one body of matter to another.
Calorie (cal): the amount of heat required to raise the temperature of 1 g of water by 1 degree C, or the amount of heat released when 1 g of water cools by 1 degree C.
kilocalories (k cal); 1 k cal= 1,000 cal. (the “calories’ on food packages).
joule (J): another unit of energy; 1 J= 0.239 cal, or 1 cal= 4.184 J.
Water’s High Specific Heat
Heat is absorbed when hydrogen bonds break
Heat is released when hydrogen bonds form
The high specific heat of water minimizes temperature fluctuations to within limits that permit life. This property is crucial for maintaining stable climates and supporting the metabolic processes of organisms.
Serves to moderate air temperature in coastal areas.
Evaporative Cooling
Evaporation cools water's surface, which is crucial for temperature regulation in organisms.
Heat of vaporization: the heat a liquid must absorb for 1 g to be converted to gas.
Evaporative cooling: helps stabilize temperatures in organisms and bodies of water.
Ice Density and Environmental Impact
Ice's crystalline structure makes it less dense, preventing oceans from freezing.
Global warming poses risks to ecosystems dependent on ice.
Water as a Solvent
Aqueous Solutions
Solutions contain a solute dissolved in a solvent (usually water).
Solution: a liquid that is a completely homogeneous mixture of substances.
Solvent: dissolving agent of a solution.
Solute: substance that is dissolved.
Aqueous solution: one in which water is the solvent.
Hydrogen shell: a sphere of water molecules that surround and interact with an ion or polar molecule, facilitating its solubility in water.
Water's polarity allows it to dissolve ionic and polar substances effectively.
Hydrophilic and Hydrophobic Substances
Hydrophilic substance: one that has an affinity for water.
Hydrophobic substance: one that does not have an affinity for water.
Oil - hydrophobic (non-polar bonds)
Acidic and Basic Conditions
pH Scale
pH measures acidity/basicity; biological fluids typically range from 6 to 8.
Acids: Increase hydrogen ion concentration.
Acid solutions have pH values less than 7.
Bases: Decrease hydrogen ion concentration.
Basic solutions have pH values greater than 7.
Most biological fluids have pH values in the range of 6 to 8.
Buffers
Buffers resist changes in pH, vital for maintaining stable conditions in living organisms.
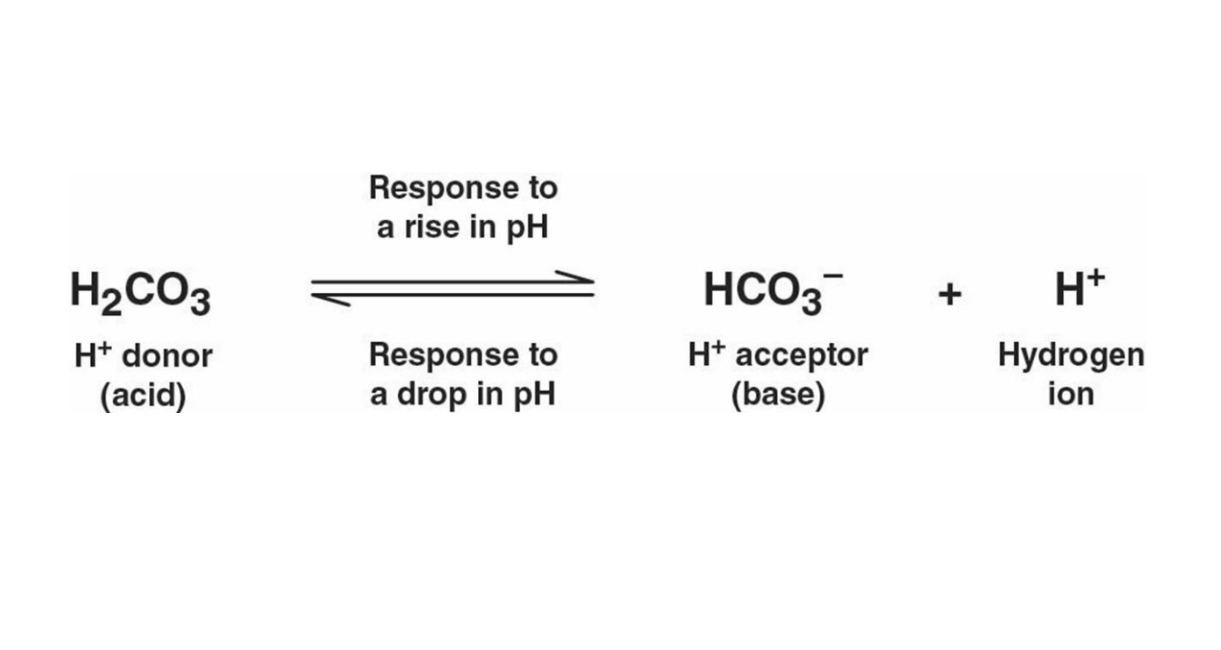
Acidification
Ocean acidification: a reduction in the pH of the ocean over an extended period of time, caused primarily by CO2 dissolved in seawater which then forms carbonic acid.
H2CO3 is the result of carbon dioxide and water, it is an acid.
As seawater acidifies, the hydrogen ions combine with carbonate ions to produce bicarbonate ions. This is a significant concern for marine ecosystems, as it reduces the availability of carbonate ions necessary for organisms like corals and shellfish to form their calcium carbonate structures.
Environmental Concerns
Ocean acidification from increased CO2 levels affects marine life, particularly organisms requiring calcium carbonate for structure.