CHAPTER 18: CHEMISTRY OF THE ENVIRONMENT
Earth’s Atmosphere
- The temperature of the atmosphere varies with altitude and the atmosphere is divided into four regions based on this temperature profile. Just above the surface, in the troposphere, the temperature normally decreases with increasing altitude, reaching a minimum of about 215 K at about 10 km.
- Commercial jet aircraft typically fly about 10 km (33,000 ft) above Earth, an altitude that defines the upper limit of the troposphere, which we call the tropopause.
- The region from 10 km to 50 km is the stratosphere, and above it are the mesosphere and thermosphere.
Composition of the Atmosphere
[ ] Sunlight and energetic particles bombard Earth's atmosphere. This energy bombardment has significant chemical and physical impacts, especially above 80 km. Due to Earth's gravitational field, heavier atoms and molecules sink, leaving lighter ones at the top. Thus, 75% of the atmosphere's mass is in the troposphere. These variables cause atmospheric composition to vary.
\n Photochemical Reactions in the Atmosphere
As the bombarding radiation passes through the upper atmosphere, it causes two kinds of chemical changes: photodissociation and photoionization. These processes protect us from high-energy radiation by absorbing most of the radiation before it reaches the troposphere. If it were not for these photochemical processes, plant and animal life as we know it could not exist on Earth.
The rupture of a chemical bond resulting from absorption of a photon by a molecule is called photodissociation. No ions are formed when the bond between two atoms is cleaved by photodissociation. Instead, half the bonding electrons stay with one atom and half stay with the other atom. The result is two electrically neutral particles.
The electrons in the upper atmosphere result mainly from photoionization, which occurs when a molecule in the upper atmosphere absorbs solar radiation and the absorbed energy causes an electron to be ejected from the molecule. The molecule then becomes a positively charged ion.
Ozone in the Stratosphere
is a layer of ozone molecules in the Earth's atmosphere that absorbs harmful ultraviolet radiation from the sun. It is important for protecting life on Earth from the damaging effects of UV radiation. Ozone is created when oxygen molecules are broken apart by UV radiation and then recombine to form ozone molecules.
The first and third processes are photochemical; they use a solar photon to initiate a chemical reaction. The second and fourth are exothermic chemical reactions. The net result of the four reactions is a cycle in which solar radiant energy is converted into thermal energy. The ozone cycle in the stratosphere is responsible for the rise in temperature that reaches its maximum at the stratopause.
Human Activities and Earth’s Atmosphere
The Ozone Layer and Its Depletion
- [ ] Ozone hole - is a region of the Earth's stratosphere where the concentration of ozone is much lower than normal. It is caused by the release of certain chemicals, such as chlorofluorocarbons, into the atmosphere.
- [ ] Chlorofluorocarbons - are a group of chemicals that contain chlorine, fluorine, and carbon atoms. They are used in a variety of products, such as aerosol sprays, refrigerants, and solvents. CFCs are known to be harmful to the environment, as they deplete the ozone layer and contribute to global warming.
Sulfur Compounds and Acid Rain
- Sulfur compoundsare compounds that contain sulfur, such as sulfur dioxide and sulfuric acid. These compounds can be released into the atmosphere through the burning of fossil fuels, and can react with other compounds in the atmosphere to form acid rain. \n
- Acid rain is rain that has a pH level lower than 5.6, and can cause damage to plants, animals, and buildings.
Nitrogen Oxides and Photochemical Smog
- Nitrogen oxides are primary components of smog, a phenomenon with which city dwellers are all too familiar. The term smog refers to the pollution condition that occurs in certain urban environments when weather conditions produce a relatively stagnant air mass.
- Photochemical smog is produced largely by the action of sunlight on vehicle exhaust gases.
Greenhouse Gases: Water Vapor, Carbon Dioxide, and Climate
- The influence of H2O, CO2, and certain other atmospheric gases on Earth’s temperature is called the greenhouse effect because in trapping infrared radiation these gases act much like the glass of a greenhouse. The gases themselves are called greenhouse gases.
- Water vapor makes the largest contribution to the greenhouse effect.
- Carbon dioxide plays a secondary but very important role in maintaining the surface temperature.
- Scientists often use the term climate changeinstead of global warming to refer to this effect because as the Earth’s temperature increases, winds and ocean currents are affected in ways that cool some areas and warm others. \n
Earth’s Water
The Global Water Cycle
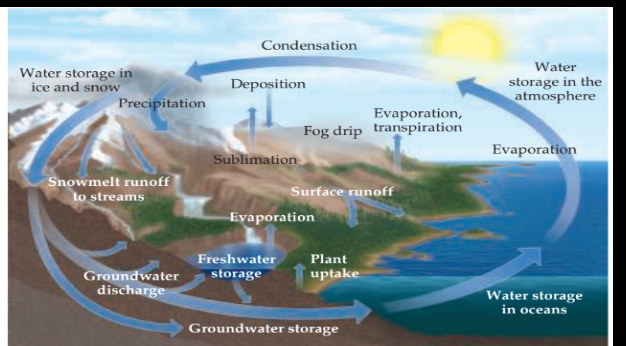
Salt Water: Earth’s Oceans and Seas
The vast layer of salty water that covers so much of the planet is in actuality one large connected body and is generally constant in composition.
Seawater is often referred to as saline water. The salinity of seawater is the mass in grams of dry salts present in 1 kg of seawater.

Freshwater and Groundwater
- Freshwater is the term used to denote natural waters that have low concentrations (less than 500 ppm) of dissolved salts and solids.
- The total amount of freshwater on Earth is not a very large fraction of the total water present.
- Approximately 20% of the world’s freshwater is under the soil, in the form of groundwater. Groundwater resides in aquifers, which are layers of porous rock that hold water.The water in aquifers can be very pure, and accessible for human consumption if near the surface.
Human Activities and Water Quality
Dissolved Oxygen and Water Quality
[ ] Biodegradable - The organic material the bacteria are able to oxidize.
Typical sources of these biodegradable materials, which are called oxygen-demanding wastes, include sewage, industrial wastes from food-processing plants and paper mills, and liquid waste from meatpacking plants.
[ ] Eutrophication - This rapid accumulation of dead and decaying plant matter in a body of water uses up the water’s oxygen supply, making the water unsuitable for aquatic animals.
Water Purification: Desalination
- The removal of salts from seawater or brackish water to make the water usable.
- Water can be separated from dissolved salts by distillation because water is a volatile substance and the salts are nonvolatile.
- Seawater can also be desalinated using reverse osmosis. \n
Water Purification: Municipal Treatment
- The water needed for domestic, agricultural, and industrial use is taken either from lakes, rivers, and underground sources or from reservoirs. Much of the water that finds its way into municipal water systems is “used” water, meaning it has already passed through one or more sewage systems or industrial plants. Consequently, this water must be treated before it is distributed to our faucets.
LifeStraw
The straw's water passes through a 100-μm textile filter and a 15-μm textile filter. Filters eliminate dirt and bacteria clusters. Next, iodine-impregnated beads eliminate bacteria, viruses, and parasites in the water. Finally, powdered activated carbon removes the iodine smell and any remaining parasites.
[ ] Trihalomethanes - are a group of chemical compounds that are formed when chlorine or other disinfectants are used to treat drinking water. They are known to be carcinogenic and can cause health problems if consumed in large amounts.
Green Chemistry
- Is an initiative that promotes the design and application of chemical products and processes that are compatible with human health and that preserve the environment.
12 principles:
- Prevention - It is better to prevent waste than to clean it up after it has been created.
- Atom Economy - Methods to make chemical compounds should be designed to maximize the incorporation of all starting atoms into the final product.
- Less Hazardous Chemical Syntheses - Wherever practical, synthetic methods should be designed to use and generate substances that possess little or no toxicity to human health and the environment.
- Design of Safer Chemicals - Chemical products should be designed to minimize toxicity and yet maintain their desired function.
- Safer Solvents and Auxiliaries - Auxiliary substances (for example, solvents, separation agents, etc.) should be used as little as possible. Those that are used should be as nontoxic as possible.
- Design for Energy Efficiency - Energy requirements of chemical processes should be recognized for their environmental and economic impacts and should be minimized. If possible, chemical reactions should be conducted at room temperature and pressure.
- Use of Renewable Feedstocks - A raw material or feedstock should be renewable whenever technically and economically practical.
- Reduction of Derivatives - Unnecessary derivatization (intermediate compound formation, temporary modification of physical/chemical processes) should be minimized or avoided if possible because such steps require additional reagents and can generate waste.
- Catalysis Catalytic reagents (as selective as possible) improve product yields within a given time and with a lower energy cost compared to noncatalytic processes and are, therefore, preferred to noncatalytic alternatives.
- Design for Degradation The end products of chemical processing should break down at the end of their useful lives into innocuous degradation products that do not persist in the environment.
- Real-Time Analysis for Pollution Prevention Analytical methods need to be developed that allow for real-time, in-process monitoring and control prior to the formation of hazardous substances.
- Inherently Safer Chemistry for Accident Prevention Reagents and solvents used in a chemical process should be chosen to minimize the potential for chemical accidents, including releases, explosions, and fires.
Supercritical Solvents
- A major area of concern in chemical processes is the use of volatile organic compounds as solvents. Generally, the solvent in which a reaction is run is not consumed in the reaction, and there are unavoidable releases of solvent into the atmosphere even in the most carefully controlled processes.
- A supercritical fluid is an unusual state of matter that has properties of both a gas and a liquid.
- Water and carbon dioxide are the two most popular choices as supercritical fluid solvents. \n