CH. 4: Reaction & Energy
Index
- 4.1 - Combustion
- 4.2 - Decomposition
- 4.3 - Energy & Environment
- 4.4 - Excess & Limiting Reactant
- 4.5 - Energy, Rate of a Reaction
4.1 Combustion
Learning objectives
- [ ] You will learn to recognise a combustion reaction, the three conditions for combustion and how you can prevent or fight a fire.
- [ ] You will learn the difference between complete and incomplete combustion and which reaction products are formed.
- [ ] You will learn to write the chemical equation of a combustion reaction.
Characteristics of combustion reactions
When a campfire is about to go out, throw a few more blocks of wood on it. The fire is produced by combustion: an exothermic chemical reaction. Chemical energy, which was previously still in the fuel, is released in the form of heat and light. At a campfire you see smoke and flames, but this does not apply to every combustion.
There are three conditions for a combustion reaction. You need a combustible substance, there must be oxygen to react with and the temperature must be high enough. The fire triangle below shows this. The reaction temperature is also called the ignition temperature. To reach the ignition temperature, for example, you must first light the burner. So much heat is released that the gas continues to burn by itself.
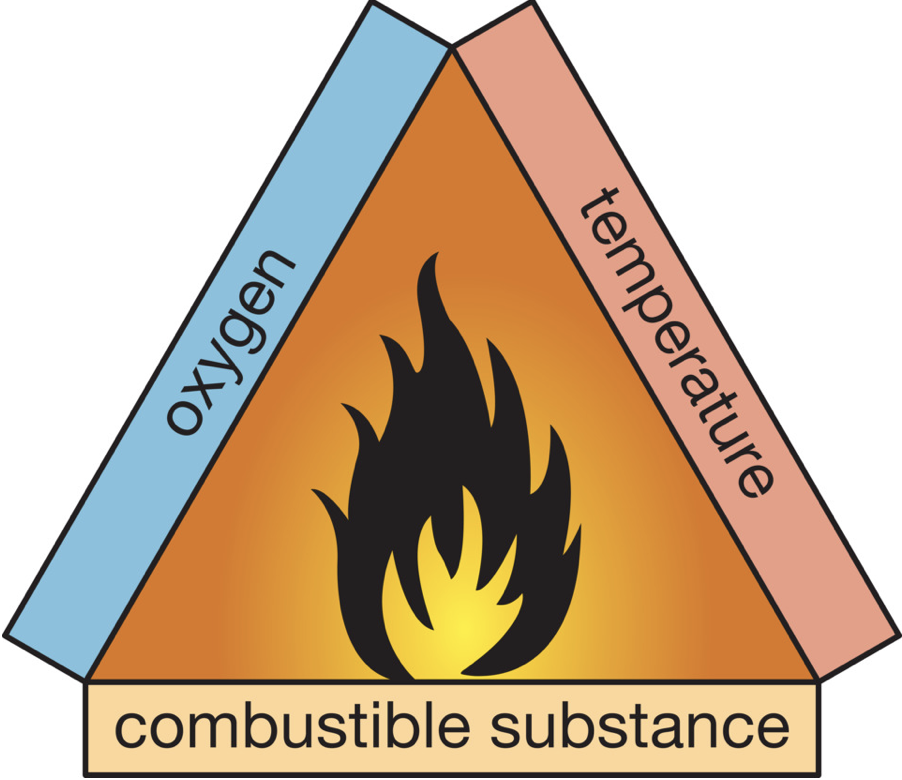
Preventing and Fighting a Fire
Many materials such as wood and plastic are combustible. With oxygen and a sufficiently high temperature they can spontaneously catch fire.
You can prevent or fight a fire by removing at least one of the three conditions for combustion. For example, you can extinguish a fire in a trashcan by putting a fire blanket over it, so there is no more oxygen getting to it.
Identifying Reaction Products in Combustion Reactions
A reagent is a substance with which you can identify another substance. An example of a reagent is clear lime water. This turns turbid when you blow carbon dioxide through it. This does not happen with other substances, so clear limewater is a suitable reagent to identify carbon dioxide.
When carbon is combusted, a colourless gas is formed. If you pass this gas through clear limewater it will become turbid. During the combustion of other substances, a compound with oxygen atoms is formed as a reaction product. For example, if you burn hydrogen, a substance is formed; water.
Most fuels consist of more than one kind of atom. Methane consists of carbon and hydrogen atoms. As a result, when methane is combusted you can detect both carbon dioxide and water as reaction products. Not only with methane, but also with the combustion of other compounds, atoms from the fuel will bond with oxygen atoms.
Complete and Incomplete Combustion
When a burner’s air control is open, it burns with a colourless or blue flame. Sufficient oxygen is present and water and carbon dioxide are formed. This is what we call a complete combustion. If you close the air control, it burns with a yellow flame and everything that touches it turns black. Because insufficient oxygen is supplied, black soot is formed in addition to water. The soot gives the flame a yellow colour, this is called an incomplete combustion.
Incomplete combustion can also produce the highly toxic gas carbon monoxide. You cannot see or smell this gas, but if inhaled, you can become unconscious and eventually even die.
Depending on the type of atoms in the fuel, the following reaction products are formed during complete and combustion.
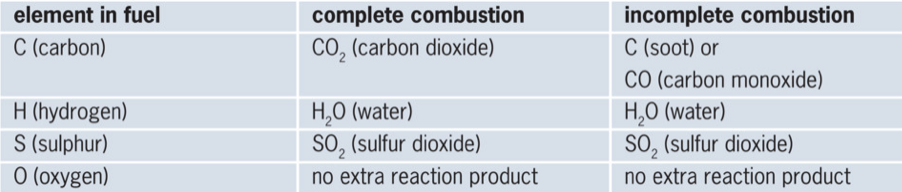
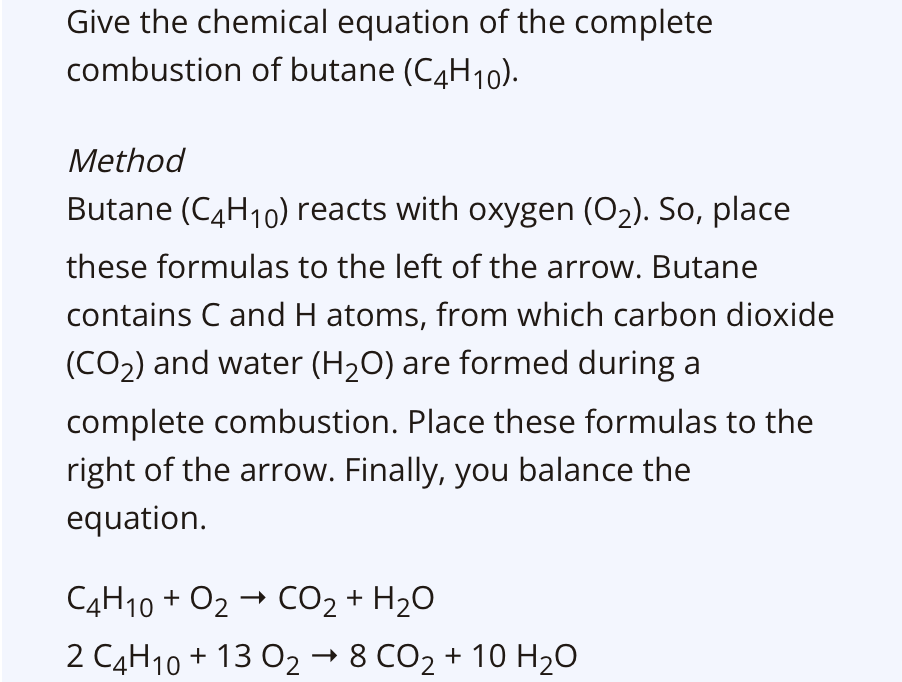
Make chemical equations of combustion reactions
During combustion, a combustible substance reacts with oxygen. So, you al ways place the formulas of these two substances on the left of the arrow in the chemical equation. The table above helps with checking which reaction products will be formed. Those formulas go on the right of the arrow. If there is another element in the fuel, the reaction product is given. Lastly you balance the chemical equation by adjusting the coefficients. The coefficient of Oxygen is the last one to be adapted.

Summary
4.4 Excess and Limiting Reactant
Learning objectives
- [ ] You will learn about an excess and a limiting reactant in a chemical reaction
- [ ] You will learn how to perform calculations based on reactions where one of the reactants is in excess
Excess and Limiting Reactant
A reaction always stops when one of the reactants runs out. Substances react with each other in a fixed mass ratio. If you mix the reactants in a different mass ratio, only one reactant will react completely. This is the limiting reactant. A part of the other reactant(s) remains, this is the excess.
Calculating with Excess and Limiting Reactant
If in a reaction, substances are not in the correct mass ratio, you can calculate which substance is in excess. You then calculate with the mass of one substance, how much of the other substance is needed. You compare the calculated amount with the given amount of said substance.
Example:
If you heat ==copper oxide== in the presence of @@methane@@, pure copper is formed, among %%other things%%: \n 4 ==CuO== (s) + @@CH4@@ (g) → 4 Cu(s) + %%CO2%% (g) + 2 %%H2O%% (g)
You heat 25 g of copper oxide in the presence of 5.5 g methane. The mass ratio of copper oxide and methane is 19.9 : 1.00. Calculate which substance is in excess and how many grams of that substance remain afterwards.
\n 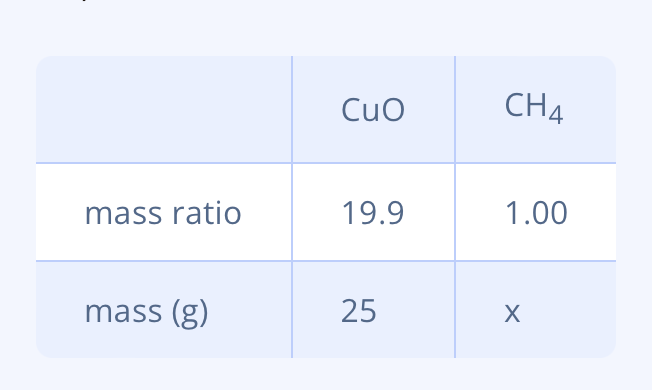
\n
/