‘Titration’ is a process with two uses:
To find exactly how much of an acid is needed to neutralise an alkali
To find the concentration of a solution
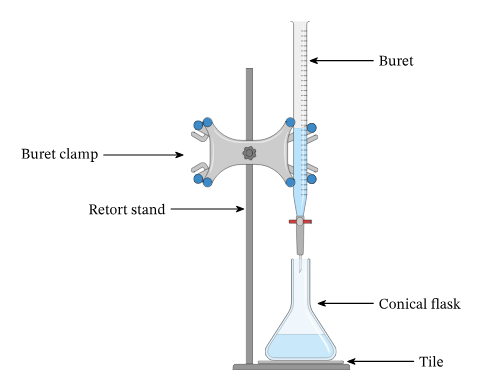
Equipment for a titration:
clamp stand, clamp and boss
conical flask
volumetric pipette
pipette filler
burette
indicator
25cm³ alkali with known concentration
acid with unknown concentration
white tile
funnel
Method:
Set up a clamp stand to hold a burette with the tap suspended above a conical flask on a white tile (for better view of colour change)
Rinse the burette and the pipette with distilled water
Use the pipette and pipette filler to measure 25cm³ of alkali into the conical flask. Make sure to read from the bottom of the meniscus
Add an indicator (such as methyl orange) to the alkali
Fill the pipette with acid using a funnel
Add drops of acid into the alkali until the indicator changes colour, showing neutralisation
 Titration calculation:
Titration calculation:

 Note
Note Studied by 5 people
Studied by 5 people Note
Note Studied by 19 people
Studied by 19 people Note
Note Studied by 7 people
Studied by 7 people Note
Note Studied by 18 people
Studied by 18 people Note
Note Studied by 6628 people
Studied by 6628 people Note
Note Studied by 55 people
Studied by 55 people Knowt
Knowt Titration calculation:
Titration calculation: