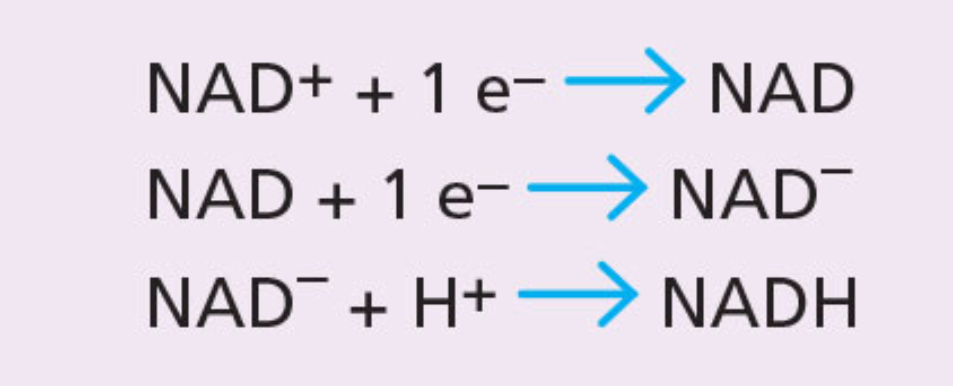Chapter 9 & 10: Enzymes
An enzyme is a biological catalyst that is made of protein.
A catalyst is a substance that speeds up a reaction, without itself being used up in the reaction.
A substrate us the substance on which an enzyme reacts.
The product is the substance(s) the enzyme forms.
e.g Amylase → Starch = Maltose
Enzymes are large molecules that are highly folded into a 3D shape.
Enzymes have areas along their surface called active sites. It is these that a particular substrate becomes attached. These make the enzyme specific.
Enzyme reactions are reversible. This means that an enzyme’s reaction can be anabolic and catabolic. Examples of chemical reactions controlled by enzymes are:
Respiration, Photosynthesis, Digestion, Protein Synthesis, and DNA Replication.
Anabolic Enzymes
DNA Ligase
Used in genetic engineering, joins two pieces of DNA together.DNA Polymerase
An enzyme that repairs and forms DNA.
Enzymes involved in photosynthesis are also anabolic because they convert carbon dioxide and water into glucose.
Catabolic Enzymes
Amylase
An enzyme that converts starch into maltose and seed germination.Catalase
Converts hydrogen peroxide into water and oxygen.
Factors Affecting Enzyme Activity
pH
Enzyme shape changes when the pH changes. If the pH becomes too high or low the enzyme changes shape (denatured).
Temperature
At low temperatures enzyme activity is reduced. An increase in temperature increases enzymes activity.
Human enzymes are designed to work at 37OC while plants work at 20-20OC.
Above a certain temperature, enzymes change their 3D shape - as a result the rate of the reaction falls.
Denaturing Enzymes
Active sites are destroyed when denaturing occurs, substrates cannot attach and enzymes cannot function. Denaturing cannot be reversed.
Immobilised Enzymes
Enzymes that are attached or fixed to each other or to an inert material.
Bioprocessing
Use of enzyme-controlled reactions to produce a product.
Bioreactor
Vessel or container in which living cells or their products are used to make a product.
Bioprocessing used to involve the use of micro-organisms such as yeast and bacteria to produce foodstuffs such as cheese, yoghurts, breads, beers and wines.
It has be developed to produce a vast range of products such as antibiotics, drugs, vaccines, vitamins, amino acids and perfumes.
Methods of Immobilising Enzymes
Attaching them to one another
Attaching them to insoluble supports (Adsorption)
Enclosing them in a membrane or gel
Adsorption
Enzymes are physically attached to inert supports, such as glass beads, ceramics, cellulose particles or artificial polymers.
In the case of trapping the enzyme in gel, sodium alginate is often used as the gel. It is prepared from algae and is permeable to the entry of the substrate and exit of the product, while the enzyme cannot leave the gel.
Advantages of Immobilised Enzymes
Enzymes can be reused which lowers costs.
Enzymes remain in the reaction vessel at the end of the process, which eans the product does not have to be separated from the enzyme.
It often increases enzyme stability.
Uses of Immobilised Enzymes
Fructose in soft drinks is converted from glucose using the immobilised enzyme glucose isomerase.
New antibiotics are created from penicillin using the immobilised enzyme oenicilin acylase.
Glucose and galactose are created from lactose using the immobilised enzyme lactase.
Induced Fit Model
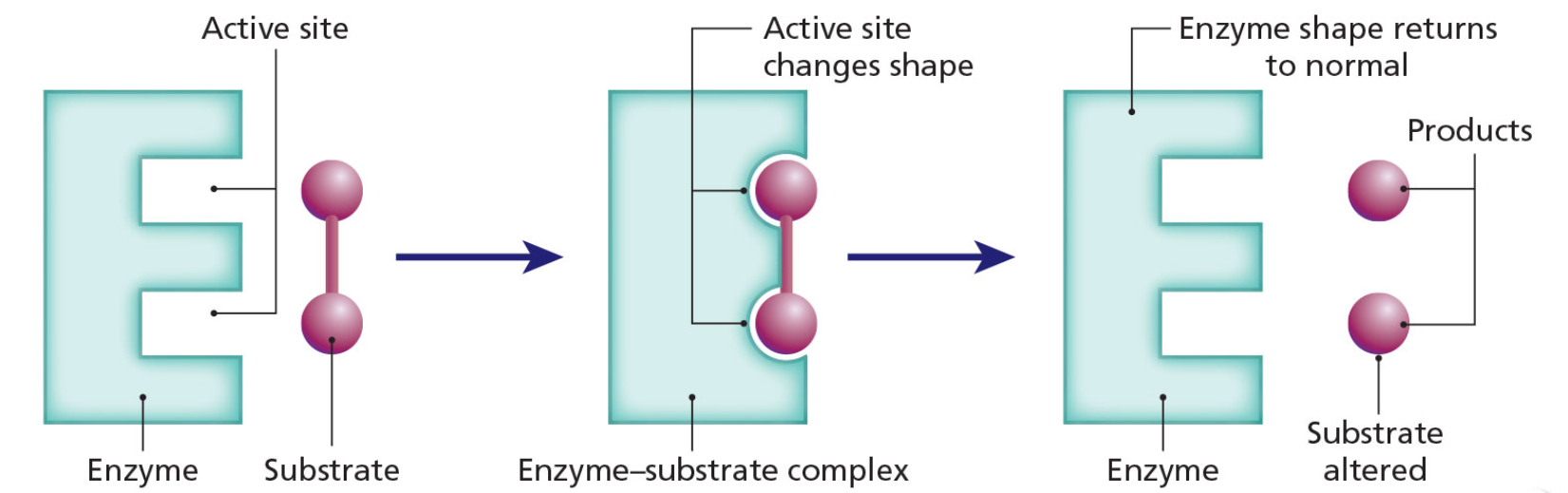
The substrate combines with the 3D active site of the enzyme.
The active site is induced to change shape slightly by the substrate.
The substrate and enzyme form an enzyme-substrate complex.
The bonds in the substrate are altered so that the substrate changes into the products.
Enzyme Specifity
Each enzyme will react with only one particular substrate.
Optimum pH
pH value at which the enzyme works best.
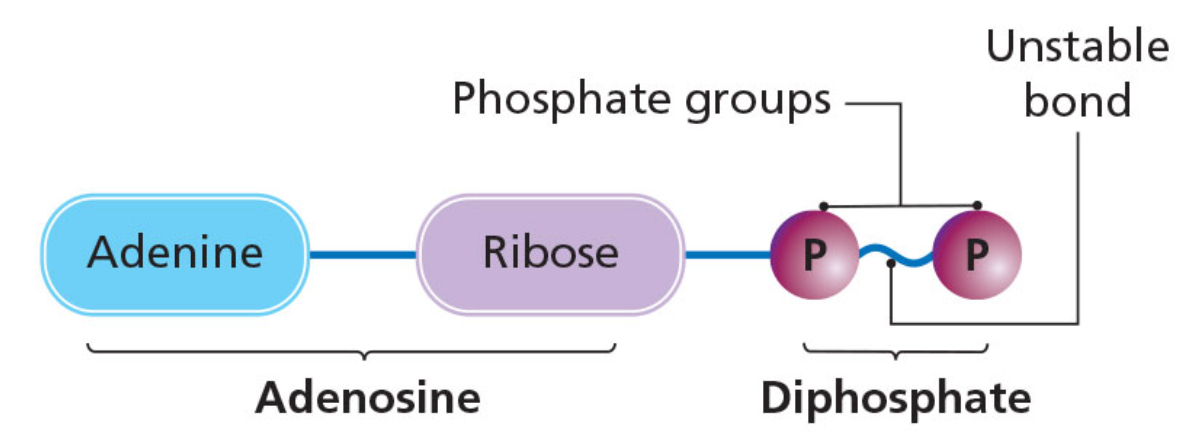
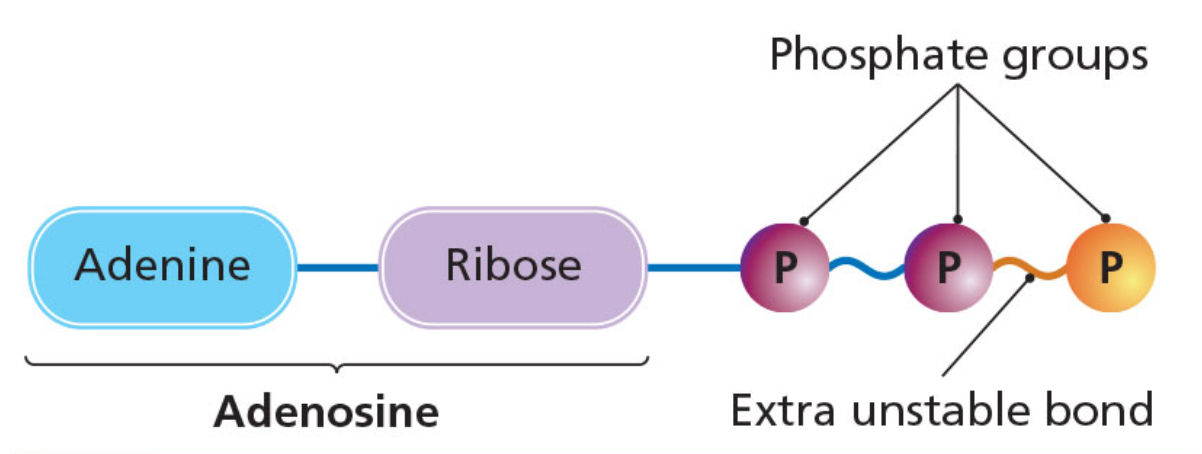
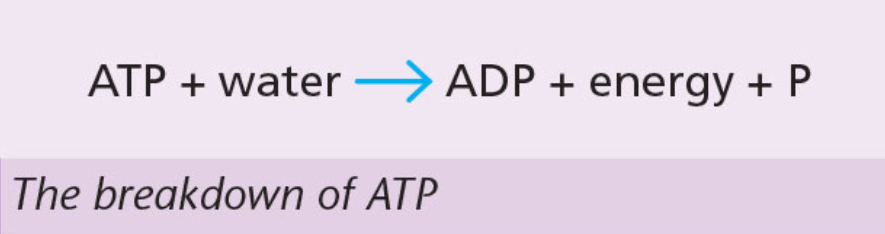
ADP and ATP
ADP (adenosine diphosphate)
made of the base adenine, a five-carbon sugar called ribose and two phosphate groups.
The bond between the two phosphate groups is unstable.
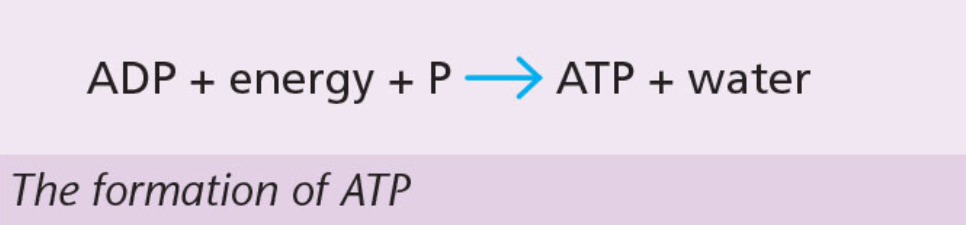
ATP (adenosine triphosphate)
Forms when another phosphate group is added to ADP.
Energy is added in the form of the unstable bond between the last posphate groups.
Process of adding a phosphate group is phosphorylation
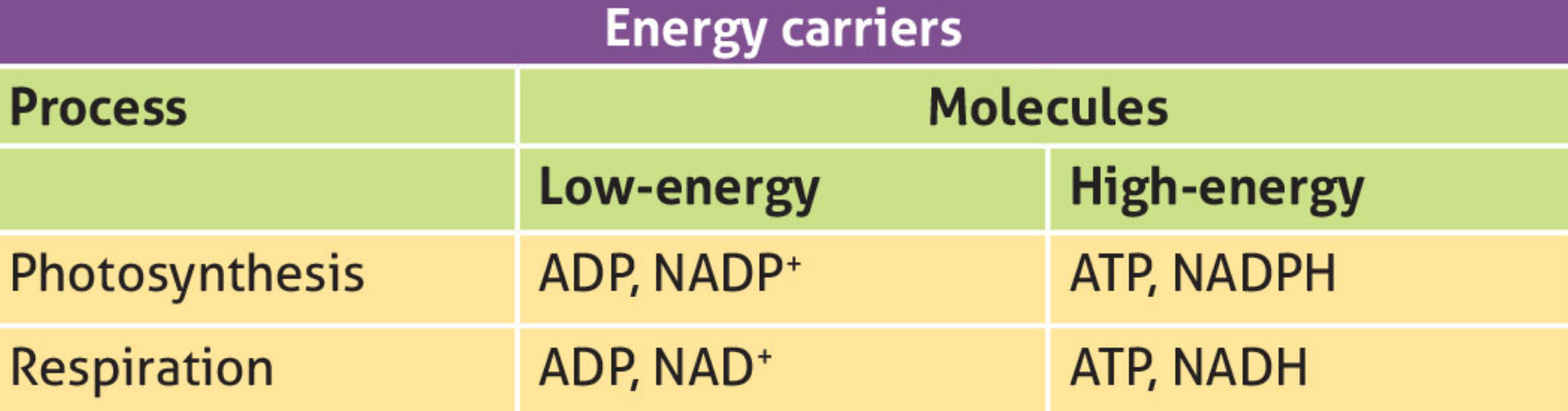
NADP+ and NADPH
NADP+ (nicotinamide adenine dinucleotide phosphate)
Low-energy molecule, involved in photosynthesis.
NADP+ can accept a pair of high energy elections (combined with a hydrogen ion or proton) to form NADPH.
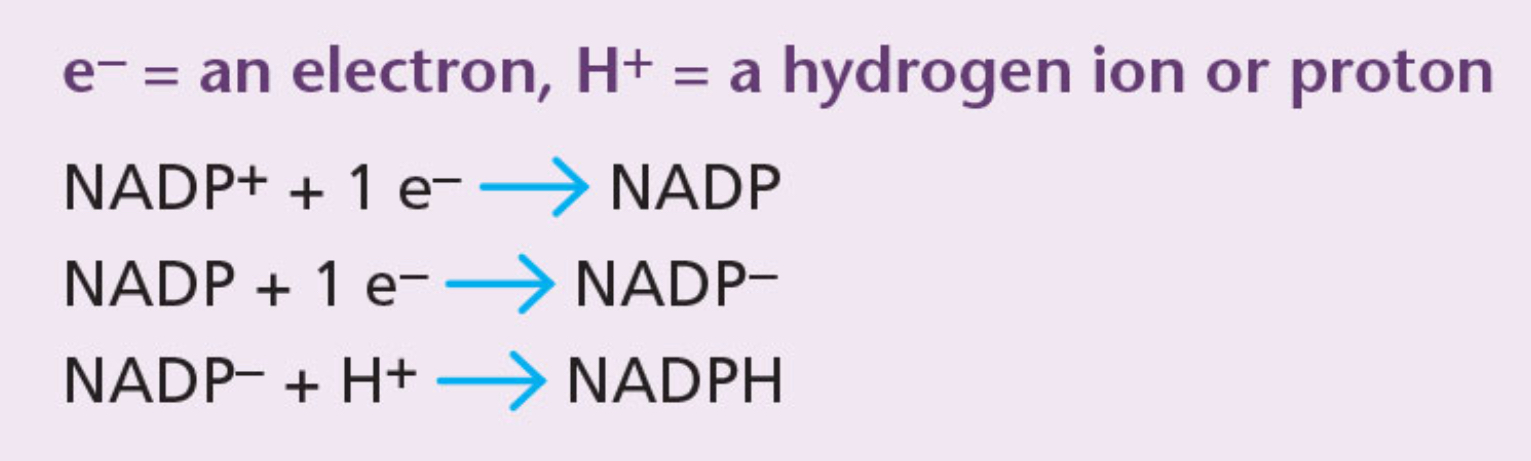
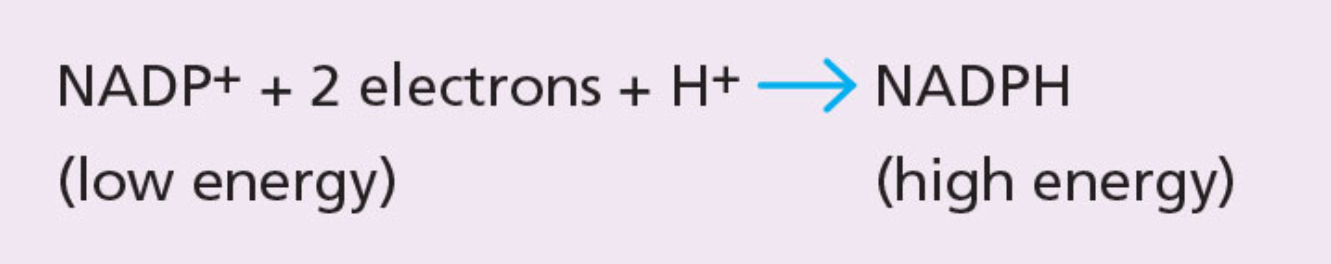
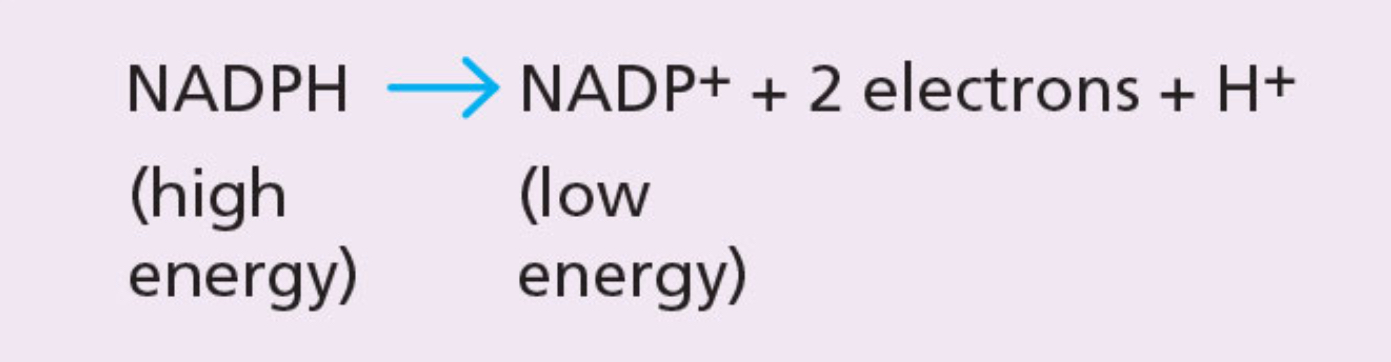
NADPH
Electron and hydrogen carrier. The energy and hydrogen it contains is used in photosynthesis to form glucose.
NAD+ and NADH
NAD+ is used in respiration as an equivalent low energy molecure to NADP+
NADH is used as an equivalent high energy molecule to NADPH.