L1: Intro to polymers
Learning Outcomes
Understand the basic concepts of polymers, including monomers, polymerization, and polymer length.
Explain the relationship between polymer length, molar mass, and properties.
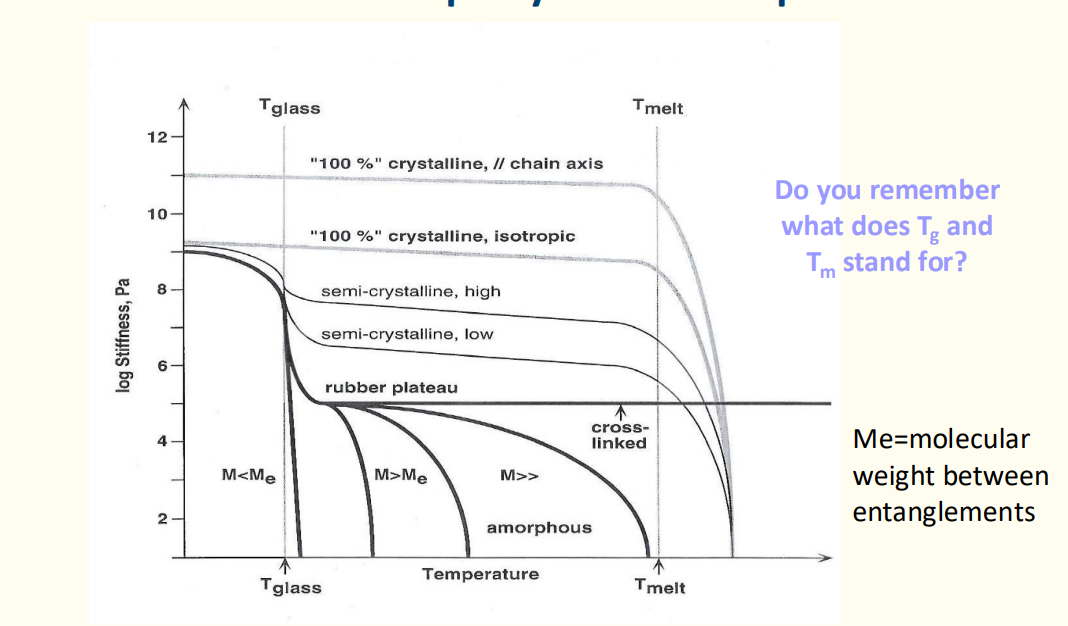
Categorize polymers based on their geometry, chemical structure, and conformation.
Understand the effect of polymer length on material properties.
Identify common polymers and their applications.
1. Introduction to Polymers
Polymers are large molecules composed of repeating structural units (monomers) connected by covalent bonds.
The term "polymer" comes from the Greek words "poly" (many) and "meros" (parts).
Hermann Staudinger (1953 Nobel Prize in Chemistry) demonstrated the existence of macromolecules, laying the foundation for polymer science.
2. Polymer Length and Molar Mass
Polymer Length:
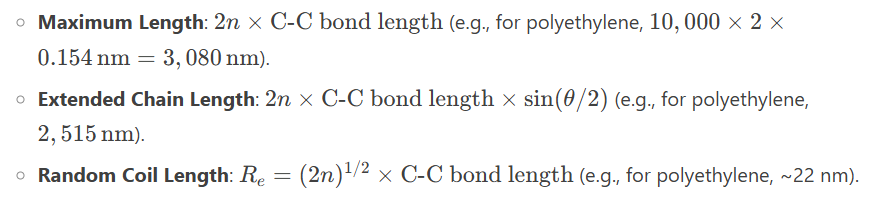
Molar Mass (MM):

Example: Polyethylene with DP = 10,000 has MM = 28 g/mol × 10,000 = 280,000 g/mol.
3. Effect of Polymer Length on Properties
Short Chains (n = 1-4): Simple gases (e.g., methane, ethane).
Medium Chains (n = 5-25): Liquids (e.g., gasoline, kerosene).
Long Chains (n = 25-50): Solids (e.g., paraffin wax).
Very Long Chains (n = 1,000-10,000): Tough plastics (e.g., polyethylene for containers).
Ultra-Long Chains (n = 100,000): High-performance materials (e.g., bullet-proof vests).
4. Polymer Chain Geometries
Linear Polymers:
Coiled: High-density polyethylene (HDPE), nylons.
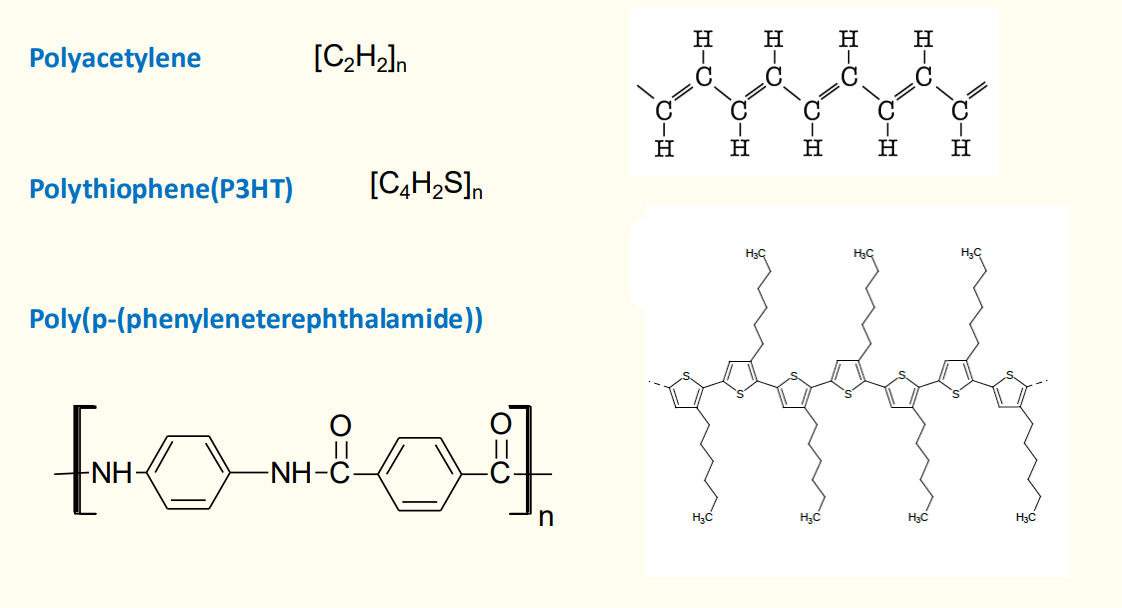
Rodlike: Poly(p-phenylene terephthalamide) (Kevlar).
Branched Polymers:
Short-Chain Branches: Linear low-density polyethylene (LLDPE).
Long-Chain Branches: Low-density polyethylene (LDPE).
Cross-Linked Polymers:
Tenuous: Rubber.
Dense: Epoxies.
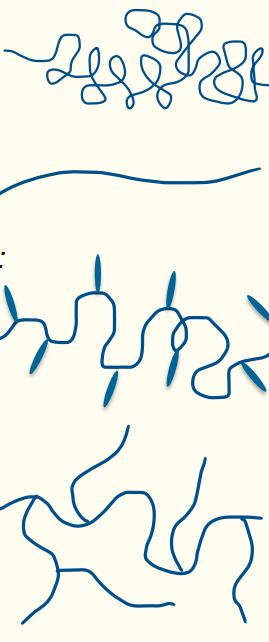
5. Polymer Conformations
Isolated Molecules:
Flexible Coil: Randomly coiled chains.
Rigid Rod: Extended, straight chains.
Entanglements:
Flexible coils and rigid rods can form entanglements, affecting material properties.
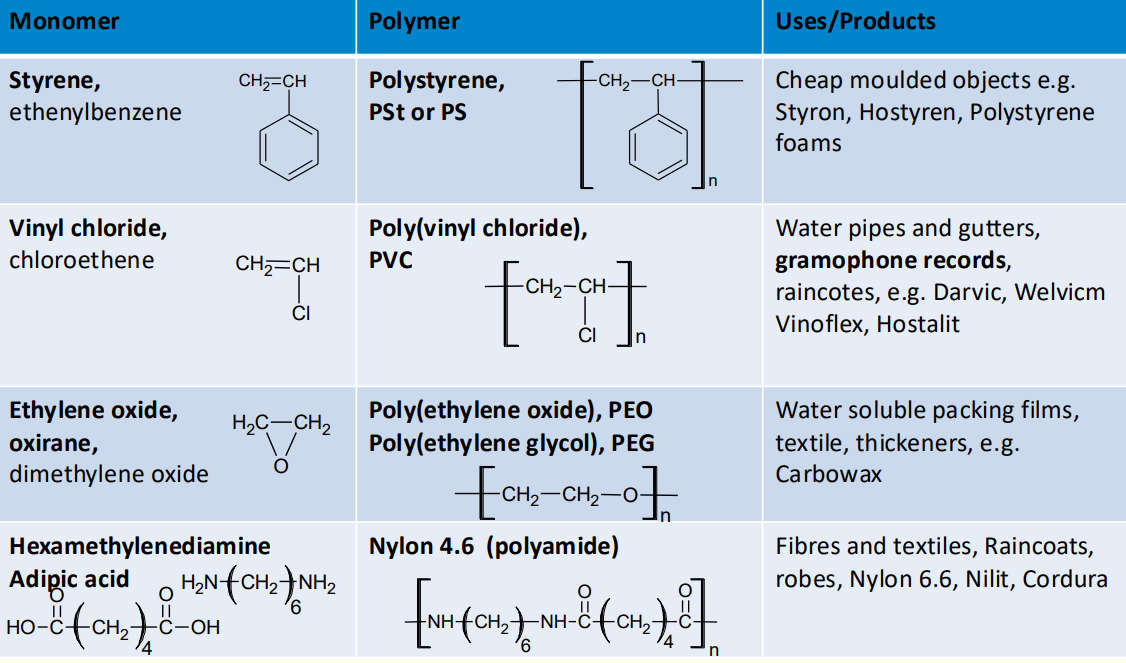
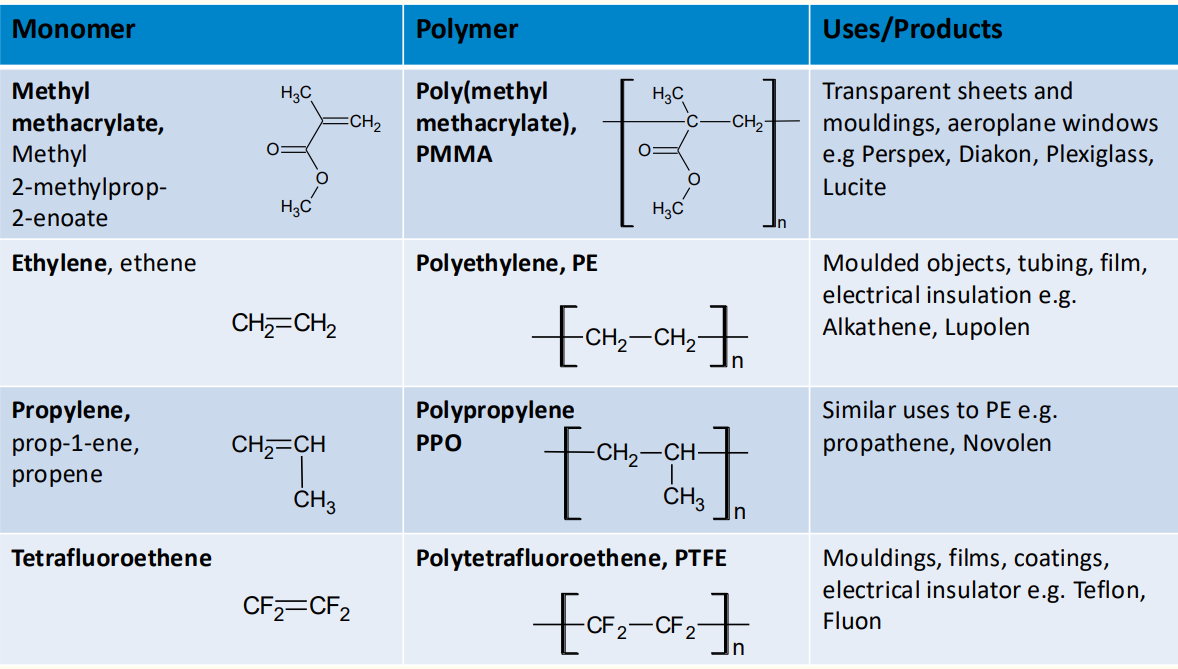
6. Common Polymers and Their Applications
Polyethylene (PE):
Used in packaging, containers, and pipes.
High-density polyethylene (HDPE) is more rigid, while low-density polyethylene (LDPE) is more flexible.
Polypropylene (PP):
Used in automotive parts, textiles, and packaging.
Polytetrafluoroethylene (PTFE):
Known as Teflon, used in non-stick coatings and electrical insulation.
Nylon (Polyamide):
Used in textiles, ropes, and engineering plastics.
Poly(methyl methacrylate) (PMMA):
Known as acrylic or Perspex, used in transparent sheets and lenses.
7. Copolymers
Regular Copolymers:
Alternating: Monomers alternate in a regular sequence (e.g., Nylon 6,6).
Block: Different blocks of monomers (e.g., diblock, triblock copolymers).
Irregular Copolymers:
Monomers are arranged randomly along the chain.
8. Summary of Key Concepts
Polymers are large molecules made up of repeating monomer units.
Polymer length (degree of polymerization) and molar mass significantly affect material properties.
Polymer geometry (linear, branched, cross-linked) and conformation (coiled, rodlike) influence mechanical and thermal properties.
Common polymers like polyethylene, polypropylene, and nylon have diverse applications in everyday life and industry