Chapter 8 Lecture Slides Tro 6th
Chapter Overview
CHEMISTRY 131, Chapter 8: Focuses on the Quantum-Mechanical Model of the Atom
Elements and the Periodic Table
Periodic Trends: Various properties of elements show trends based on their position in the Periodic Table.
Periodic Properties: Essential to understand what causes this periodicity.
Electron Arrangement:
Electrons are arranged in different energy levels or shells.
Electron Configuration:
The arrangement influences periodic properties, particularly the configuration of outer valence electrons.
The Nature of Light
Energy and Light:
Electron configurations are determined through absorption and release of energy as light.
Electromagnetic Radiation:
Defined as oscillating electric and magnetic fields traveling through space at the speed of light.
Light is characterized by its wavelength (λ) and frequency (ν):
Wavelength (λ): Distance between successive wave crests.
Frequency (ν): Number of wave crests passing a point per unit time.
Relationship: The shorter the wavelength, the higher the frequency (and vice versa) with the equation λ·ν = c, where c is the speed of light (~3.00 x 10^8 m/s).
Electromagnetic Spectrum:
Contains various types of electromagnetic radiation (radio waves, visible light, X-rays, gamma rays), each with different wavelengths and frequencies.
Spectrum is characterized by regions of increasing energy and frequency while decreasing wavelength.
Photoelectric Effect:
Observed when light hits a metal surface and ejects electrons, dependent on light frequency.
Einstein's Contribution: Described light as consisting of individual photons, each carrying energy proportional to frequency (E = hν).
Planck’s constant (h) = 6.63 x 10^-34 J·s is crucial in calculating photon energy.
Quantum Mechanics and the Atom
Heisenberg Uncertainty Principle:
It is impossible to know both the exact position and momentum of an electron precisely.
Schrodinger's Wave Functions:
Developed mathematical equations to describe electron movements and energies in orbitals.
Quantum Numbers:
Principal Quantum Number (n): Indicates the main energy level (n = 1, 2, 3, ...).
Each level corresponds to a shell; higher n values allow subshells.
Angular Momentum Quantum Number (l): Determines subshell type (values from 0 to n-1).
Magnetic Quantum Number (mₗ): Specifies the orientation of orbitals.
Spin Quantum Number (mₛ): Reflects the intrinsic spin of electrons (+1/2 or -1/2); electrons in the same orbital must have opposite spins.
Atomic Orbitals
Shapes of Orbitals:
s orbitals: Spherical (1 lobe). 3d
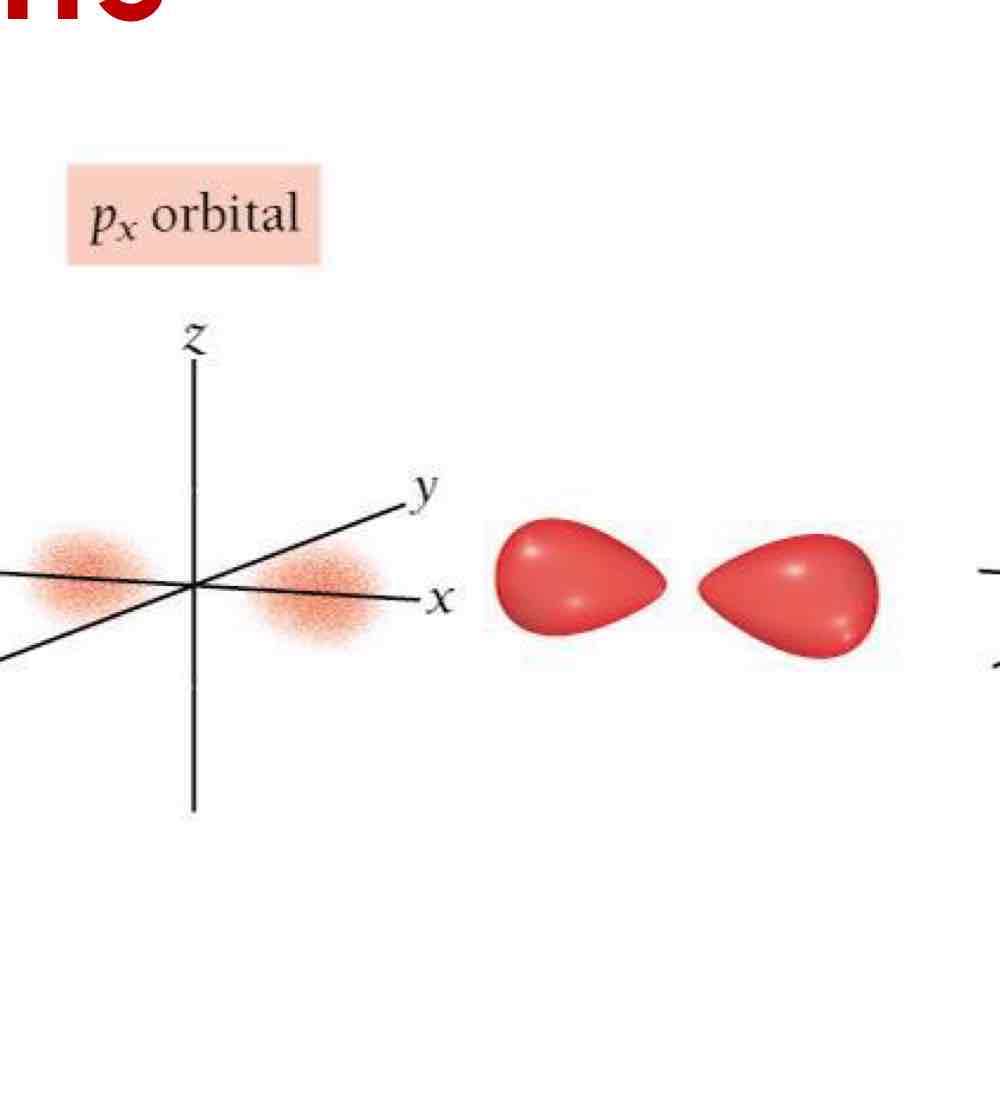
p orbitals: Dumbbell-shaped (2 lobes).
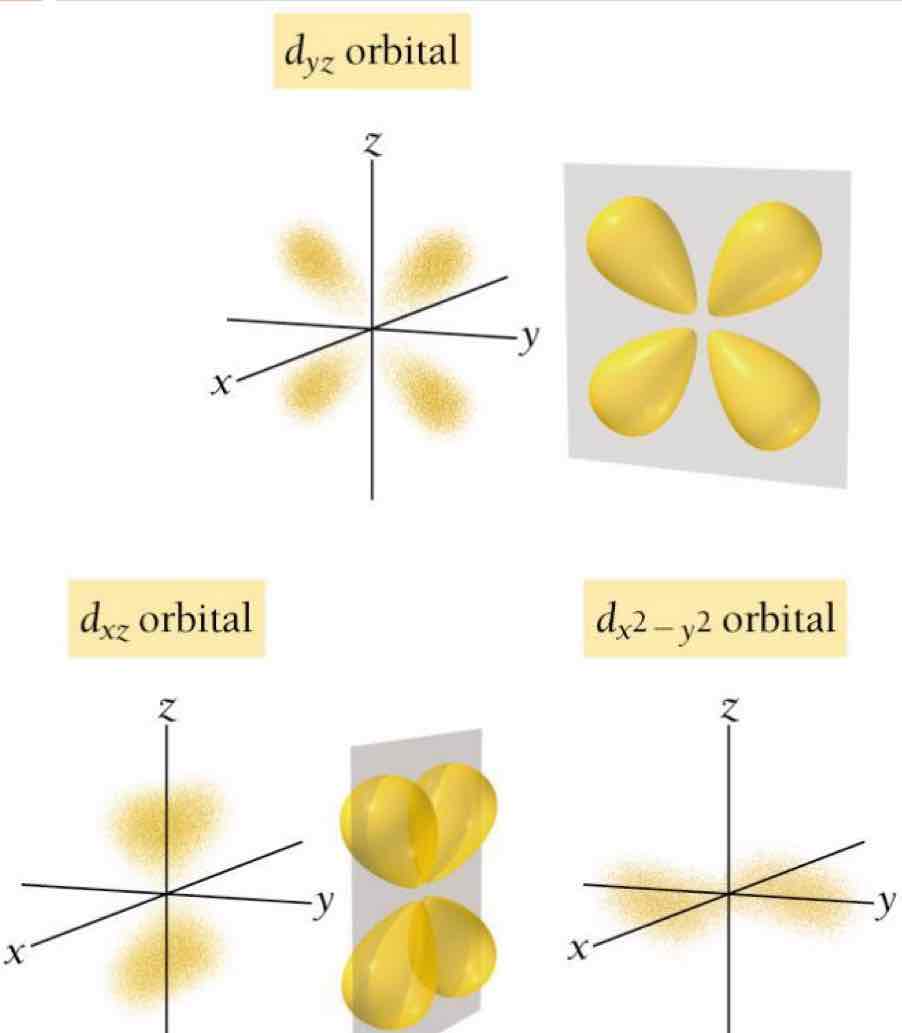
d orbitals: Double dumbbell shape (4 lobes).
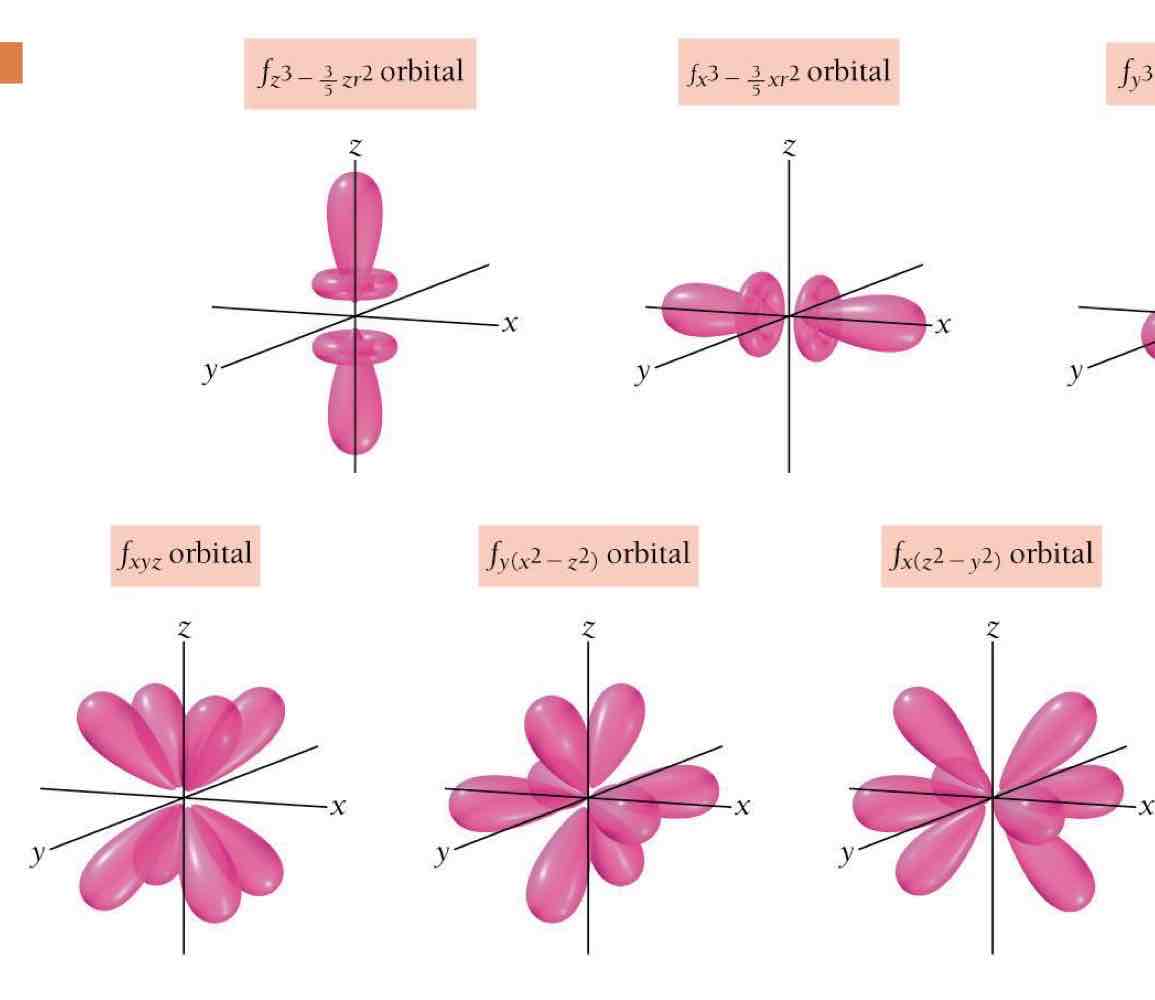
f orbitals: Quadruple dumbbell shape (8 lobes).
Electron Capacity in Orbitals:
Each orbital can hold up to two electrons, adhering to the Pauli exclusion principle.
Critical Examples and Calculations
Example Calculation: Wavelength and frequency conversions using c = λν.
Transition of electrons leading to emission or absorption of light.
Shortest wavelength corresponds to the largest energy drop in electron transitions (e.g., n=3 to n=1 is an emission).
Concluding Thoughts
The quantum mechanical model provides a complex understanding of atomic structure and behavior, highlighting the dual nature of light and electrons as both particles and waves. The developed quantum numbers guide the predictions of electron configurations, crucial in the study of chemical properties and reactions.