Intermolecular Forces
Kinetic Molecular Theory
The state of a material depends on the kinetic energy the particles possess and the strength of attraction between the particles. Particles have different types of freedom of motion that occur in different degrees.
| Type of freedom | Description | State |
|---|---|---|
| Translational | Can move from one position in space to another | Gas |
| Rotational | Can reorient in space | Liquid |
| Vibrational | Can oscillate at a particular point in space | Solid |
Kinetic energy is proportional to temperature.
Phase Changes
Gases condense when temperature decreases or the pressure increases.
Intermolecular forces between opposite charges are electrostatic forces. The larger the charge the stronger the attraction and the longer the distance the weaker the attraction.
Dispersion forces/dipole induced is temporary unequal distribution of electrons, which is temporary polarity. The magnitude of the induced dipole is influenced by the volume of the electron cloud where a larger cloud has stronger attractions and the shape of the molecule where the more surface that interacts yields a strong attraction.
Dipole-dipole attractions are the attractions between permanent polarity in molecules.
Hydrogen bonds occur when hydrogen bonds to nitrogen, fluorine, or oxygen which forms a strong dipole and is attracted very electronegative atoms. Hydrogens are exposed protons and attract neighbouring electron clouds.
Ion-dipole attraction determine if the ionic compound is water soluble.
Solubility
Like dissolves like. Miscible liquids (form homogenous solution when mixed) will always dissolve each other. Polar substances dissolve polar solvent (OH, CHO, C=O, COOH, NH2) and nonpolar substances dissolve nonpolar solvents (C-H, C-C). Polar substances are hydrophilic and nonpolar substances are hydrophobic.
Immiscible liquids describe liquids where their attractive forces are so great they remain separated. An example is trying to mix water and oil.
Boiling Points
Molecules with hydrogen will have hydrogen bonding and therefore higher boiling points than expected. Group 14 hydrides has low intermolecular forces because they are nonpolar. The polar hydrides of groups 15-17 have higher boiling points due to dispersion and dipole-dipole interactions compared to group 14.
Surface Tension
Liquids tend to minimize their surface area. Surface molecules have less neighbours attracting them and are less stable than the liquids internal molecules. The higher potential energy on a liquids surface which increases the surface area. High temperatures reduce surface tension since temperature raises the average kinetic energy of each molecule, increasing their motion. Strong intermolecular forces create high surface tension.
Viscosity
Viscosity is a liquids resistance to flow. Strong intermolecular forces create a larger resistance to flow. Spherical molecules have lower viscosity since less surface contact lowers attraction. High temperatures will decrease a liquids viscosity since it increases the average kinetic energy of the molecules allowing them to overcome flow more easily.
Capillary Action
When a liquid goes against gravity and climbs a thin tube it is called capillary action. The narrower the tube the more the liquid rises. Cohesive forces attract liquid molecules to one another while adhesive forces attract liquid molecules to the tube’s surface.
The meniscus is due to the cohesive and adhesive forces of the liquid and the tube. Concave occurs when the adhesion is stronger than the cohesion, creating a “u” shape and convex occurs when the cohesive forces are stronger than the adhesive forces, creating an “n” shape.
Vaporization
High energy molecules may be able to overcome the liquids attractive forces and leave the solution, these molecules that leave are called vapor. A higher surface area results in a higher evaporation rate. High temperatures results in high evaporation rates. Weak attractive forces cause faster rates of evaporation. Liquids that vaporize easily are volatile. Vaporization is endothermic. The heat of vaporization (∆H vap) is the heat required to vaporize one mole of liquid and is somewhat temperature depended.
Condensation
Condensation occurs when vapor molecules lose their energy and return to the liquid solution. This can occur when the vapor molecules collide with the surface of the liquid. Condensation is exothermic.
Vaporization & Condensation
∆H condensation = −∆H vaporization
Open containers vaporize molecules faster than they can condensate, resulting in a net lose of liquid.
Closed container systems will reach a dynamic equilibrium where the amount of liquid and vapor does not change. When the conditions change the system shifts to reduce the effects of the change.
The pressure exerted by the vapor onto the liquid in a closed container is called vapor pressure. Weak attractive forces create higher vapor pressures. High vapor pressures come from more volatile liquids. High temperature increases vapor pressure.
Boiling Points
A liquid reaches it boiling point when the vapor pressure is the same as the external pressure. Normal boiling points are calculated at 1 atm. Once a liquid reaches its boiling point, the temperature of the liquid stays constant. The temperature increases again when all the liquid is turned into gas.
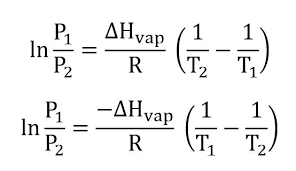
This equation creates a linear function and can be calculated from two measurements of vapor pressure and temperature
Supercritical Fluid
A supercritical fluid can be obtained in a sealed container with a critical pressure. At some critical temperature, there will be no difference meniscus and no different between the liquid and the vapor.
Sublimation and Deposition
A solid’s molecules vibrate, the process of a surface molecule leaving the solid is called sublimation. The recapturing of a molecule is called deposition. Solids and gases exist at a dynamic equilibrium and have a vapor pressure in a closed container where the temperature is below their melting point.
Melting Point
A solid melts or fuses when it reaches its melting point. At this point the attractive forces of the molecules that holds them in place are overcome. The opposite of melting is freezing. When the melting point is reached, the temperature stays the same until all of the solid is melted, then the temperature increases. Melting is endothermic while freezing is exothermic. The heat of fusion refers to the amount of energy required to melt one mole of solid.
∆H crystallization = −∆H fusion
∆H sublimation = ∆H fusion + ∆H vaporization
Phase Diagrams
Phase diagrams show state changes that occur at different pressures and temperatures. Regions represent states, lines represent state changes, and the triple point is where the molecules exists at all three states simultaneously. 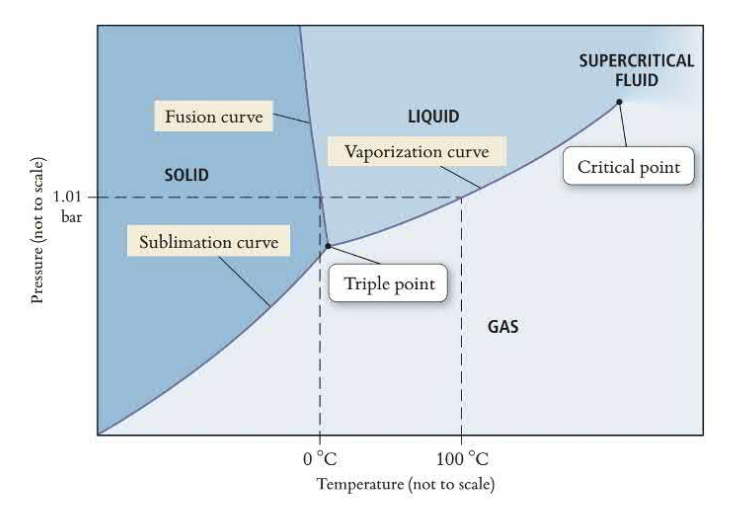
X-Ray Crystallography
X-ray diffraction enables us to figure out the atomic and molecular arrangement of atoms as well as determine their distance. Constructive and destructive interference of X-ray waves allows for an interference pattern to be created. The path length difference of the rays must be 2a where a is the diameter of the atom and a is an integral of a wavelength. 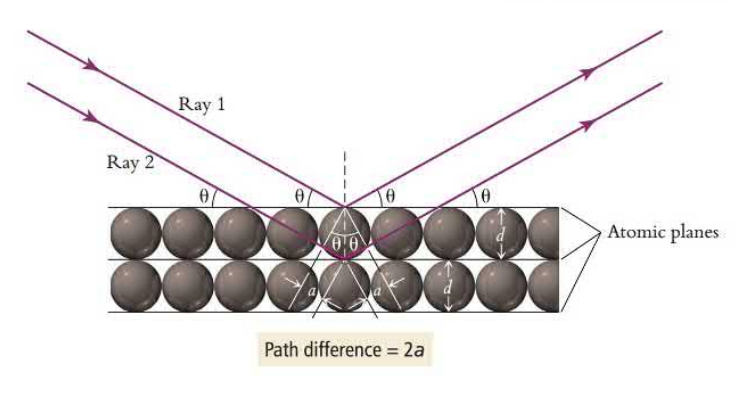
Bragg’s law is the equation that measures constructive interference so the distance between the layers can be calculated.
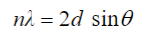
Crystal Lattice
When cooled slowly, liquid particles align themselves to maximize attractive forces. The unit cell is the smallest unit of arrangement
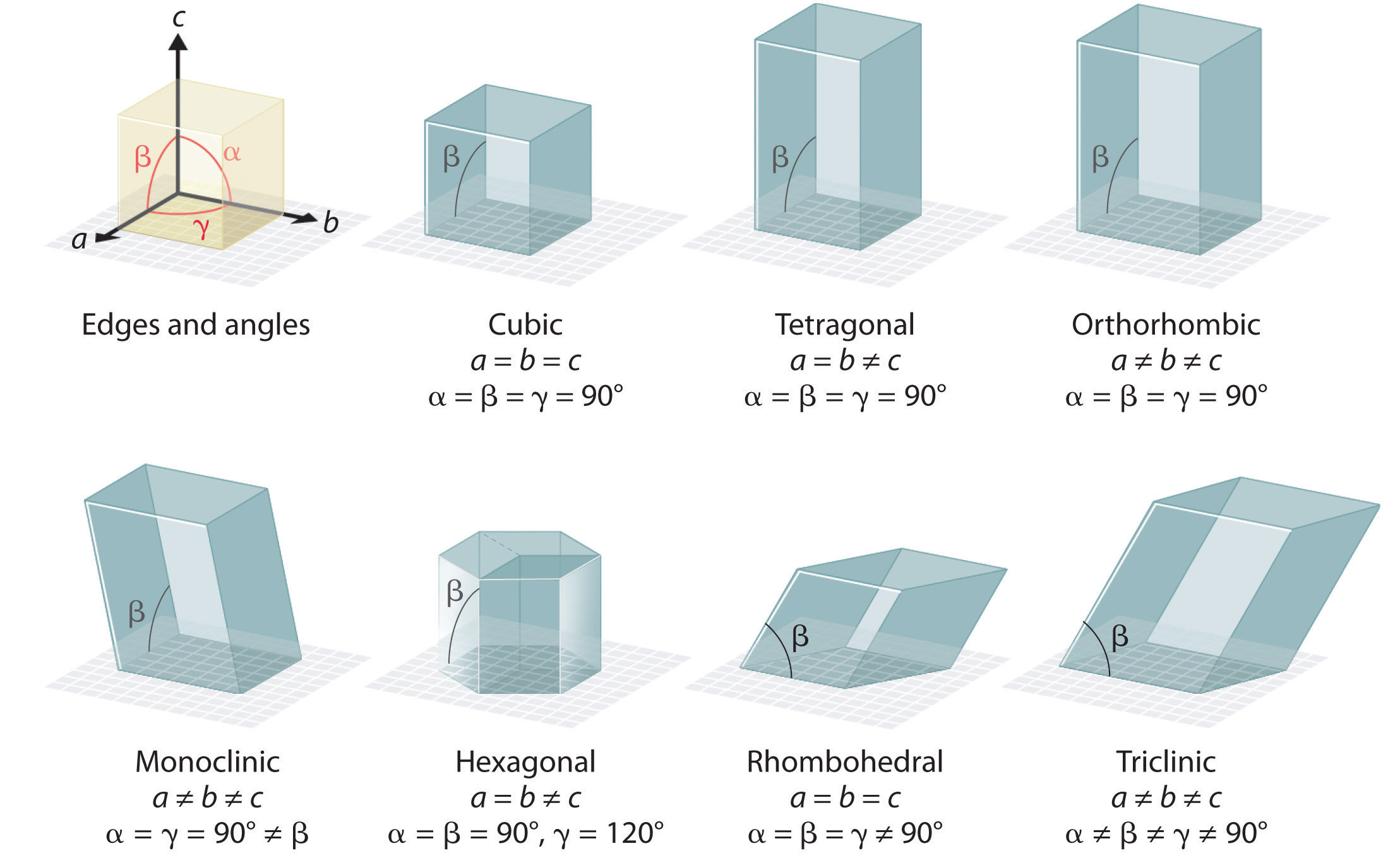
Within the unit cell, the number of atoms each atom is in contact with is the coordination number. Packing efficiency is the percentage of the unit cell occupied by particles.
Cubic Unit Cell
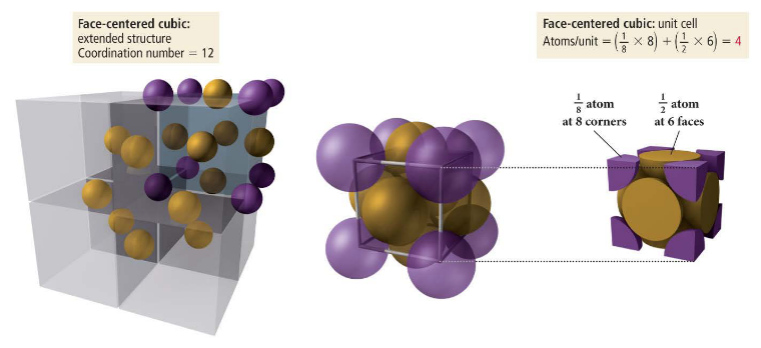
The cubic unit cell is a cute, where the particles found in the corners are 1/8th of a particle and the particles found on the face are 1/2 of a particle.
The simple cubic cell has 1/8th of a particle in each corner, it contains 1 atom total and has a coordination number of 6.
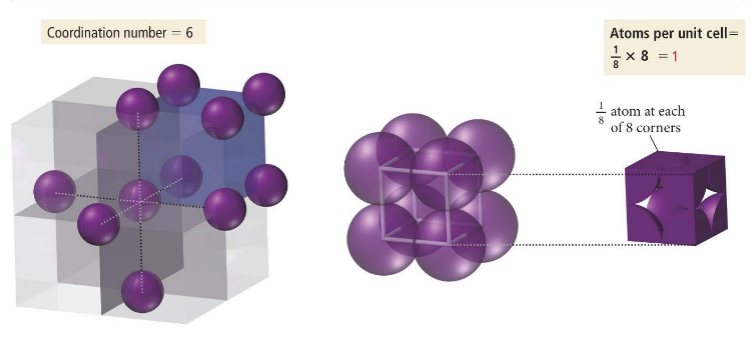
The body centered cubic cell has 1/8th of a particle in every corner and one full particle in the center. It contains 2 atoms and has a coordination number of 8. 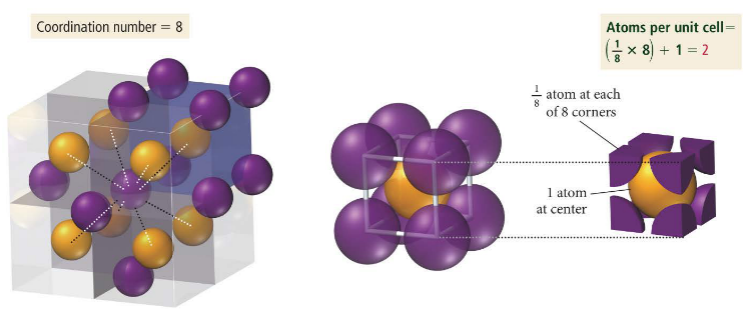
The face centered cubic cell has 1/8 of a particle in each corner and 1/2 of a particle on each face. It contains 4 atoms and has a coordination number of 12.