Chapter 16 - Cell Signaling
Cell signaling is communication between cells, not within a single cell. This is from one cell to another, and is often done via the use of chemicals called hormones. Transducing a signal within a cell is signal transduction
Insulin is a hormone, secreted by the pancreas as a response to a raise in blood sugar, which happens when there is more food digested. Insulin signals the cell to balance out levels again. Insulin has receptors on the cell (muscle and liver particularly), causing the cell to react and take in more glucose, decreasing blood levels of glucose. Insulin doesn’t enter the cell, but when bound to a receptor causes a conformational change which then has other effects on the cell, this is known as signal cascading
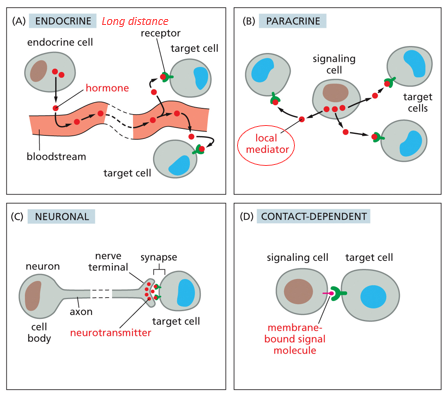
Communication is fundamental for all organisms, and how they communicate with the environment. Endocrine signaling involves hormones transmitted through blood, traveling long distances. Paracrine signaling is within a tissue, stimulating neighbor cells. Synaptic signaling is electrical/chemical and used for neuronal cells in the brain/nervous system. Contact dependent communication is between directly touching neighboring cells. Signals must be transferred quickly and selectively, only binding to specific receptors with efficiency. Proteins are highly selective/specific, which is why they are used for communication
The receptor of a cell can either be on the cell surface, or intracellularly on the nucleus. Hydrophobic molecules and steroid hormones diffuse across the membrane and bind to the nucleus directly
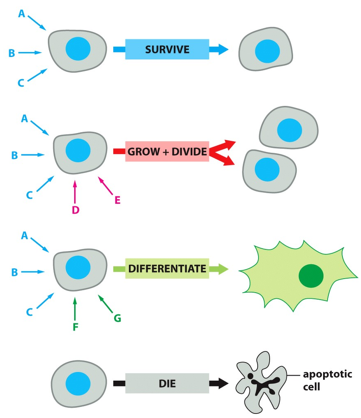
Different signals to a cell may be telling to to survive, grow and divide (with the addition of certain hormones), or to differentiate (survival signals plus differentiation specific ones). With no signals, the cell will go into apoptosis and die. If the cell isn’t actively being told to survive, it will die.
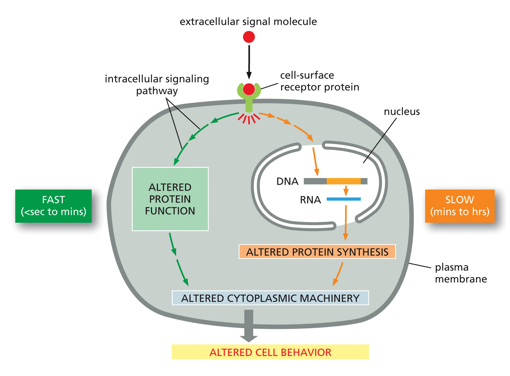
Intracellular communication (signal transduction) is mainly regulated by kinases and phosphatases, which are key enzymes in many processes. A cell may be doing one thing on its own, but if there is a signal it may follow an altered pathway very quickly, using proteins it already has to do something fast. A signal may also tell a cell to do something that it doesn’t have proteins to do immediately (like DNA manipulation and gene regulation), producing these proteins takes time so this is a slower response. Both of these alter cell behavior
Kinases often phosphorylate other kinases which do the same, causing a signaling cascade. Phosphatases are used to turn on/off these processes, removing the phosphate and breaking the cycle
The same signal may be used to induce different cellular responses in different types of cells, depending on the varying kinds of pathways attached to each receptor. Acetylcholine (Ach) does this, in the heart decreasing heart rate and inducing secretion in salivary glands. It may also cause muscle contraction in smooth muscles
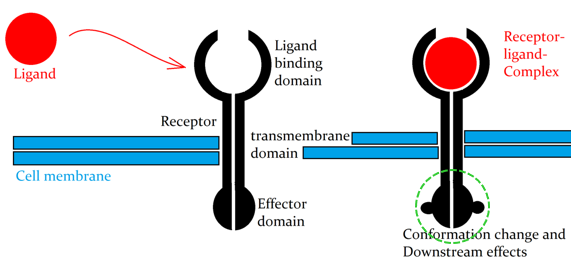
The ligand (signaling molecule) won’t cross the cell membrane itself, but rather bind to a receptor and cause a conformational change on the receptor, which often opens a new binding site. This new internal binding domain can start a cascade inside the cell. The downstream effects involve effector proteins, which may be metabolic enzymes, cytoskeletal proteins, or transcription regulators, all causing specific target-cell responses
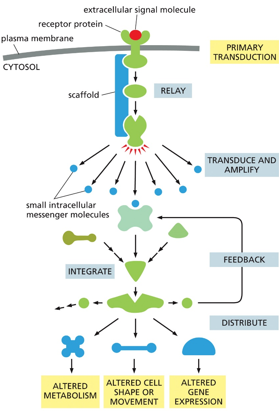
A signal cascade is modular, allowing for varying pathways from a single signal. Scaffold proteins help certain proteins bind in a sequence
Feedback regulation can be positive or negative. Positive feedback will push an extreme further and produce more and more of something, and negative feedback will decrease levels of something
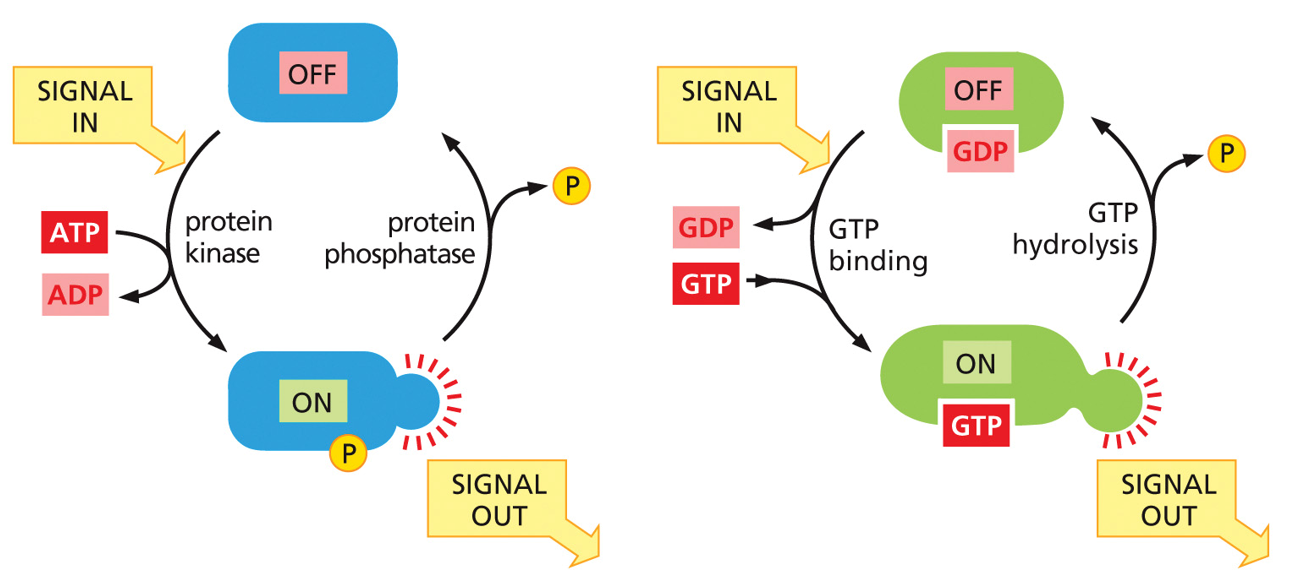
Kinases are on switches, phosphatases are off switches (WITH DEPHOSPHORYLATION). G-proteins are molecular timers, and hydrolyze their own ATP (NOT DEPHOSPHORYLATION). Each kinase has a counter phosphatase.
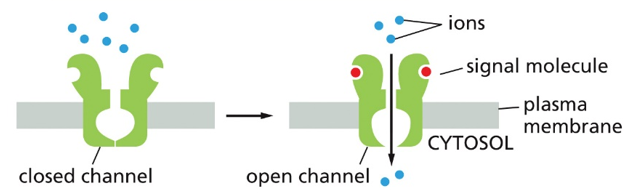
Ion channel coupled receptors will remain closed until a signal molecule binds, causing a conformation change to be open, and allow in particular ions
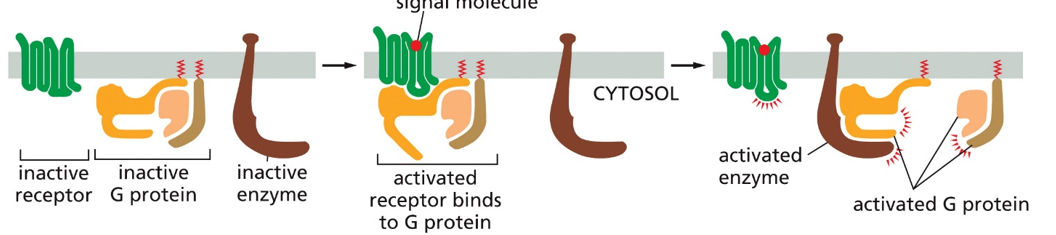
G-protein coupled receptors consist of 3 elements when inactive. These are the receptor, inactive g-protein, and inactive enzyme. When the receptor is bound by the ligand, the conformational change will allow the G-protein to bind (activating the G-protein), which then allows the G-protein to bind to an enzyme and activate it.
There are also monomeric g-proteins, that act as an alpha subunit on their own. All G-proteins are cytosolic, and the alpha subunit contains the GTP.
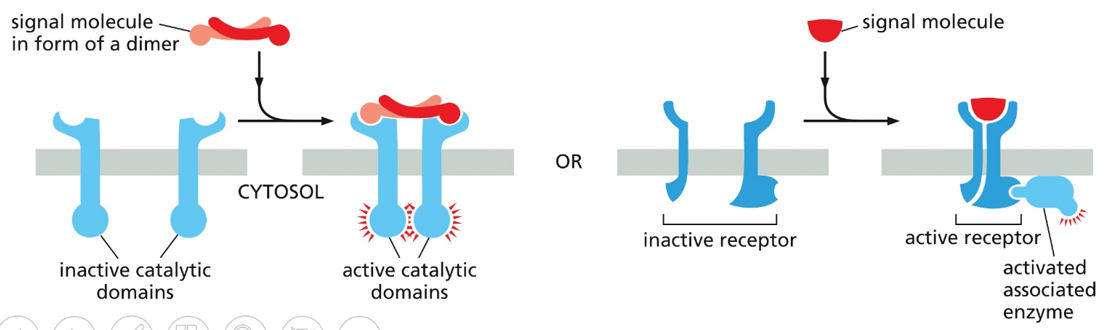
An enzyme coupled receptor will have inactive catalytic components that when bound by a molecule will cause a conformational change to bring catalytic components together and cause them to activate
Ligands can be orthosteric (primary) or allosteric (secondary)
The G-protein coupled surface receptor (GCPR) is a protein comprised of 7 helical structures, and used in cases of hormones, neurotransmitters, messengers for immune/olfactory/gustatory systems, and as a photon receptor. The 7 helixes form a sort of basket, which allows the receptor molecule to bind and cause a conformational change. All of these bind trimeric G-proteins.
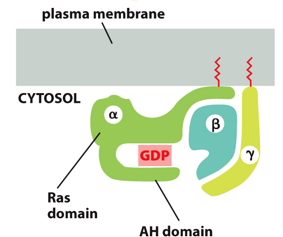
The trimeric G protein is a heterotrimer that lies within the cytosolic side of the plasma membrane. It will bind GTP when on and GDP when off, and it acts as a lipid membrane anchor. It can turn on effector proteins with the molecular times, and when activated through conformational change, changes its affinity
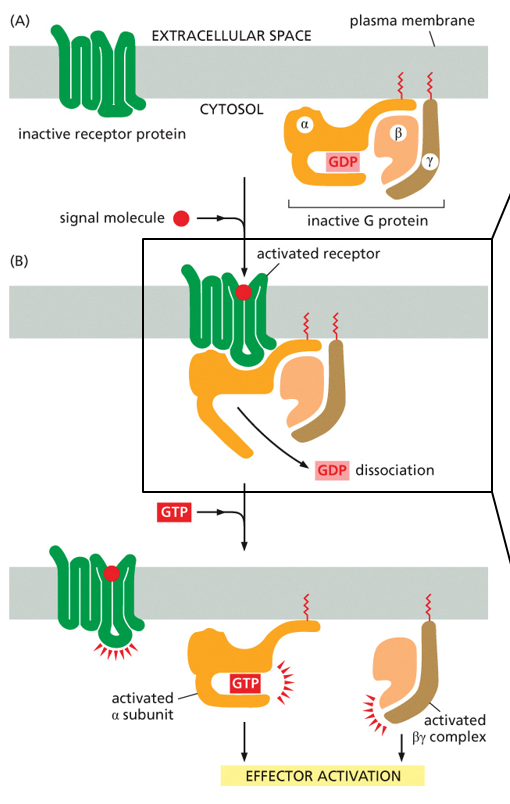
To activate it, the alpha subunit will bind with the receptor, given that the receptor has bound the ligand and changed conformational states. When this binds, the GDP dissociates and the alpha subunit can bind another GTP, becoming activated. The other subunits are beta and gamma, which bind the inactive alpha subunit.
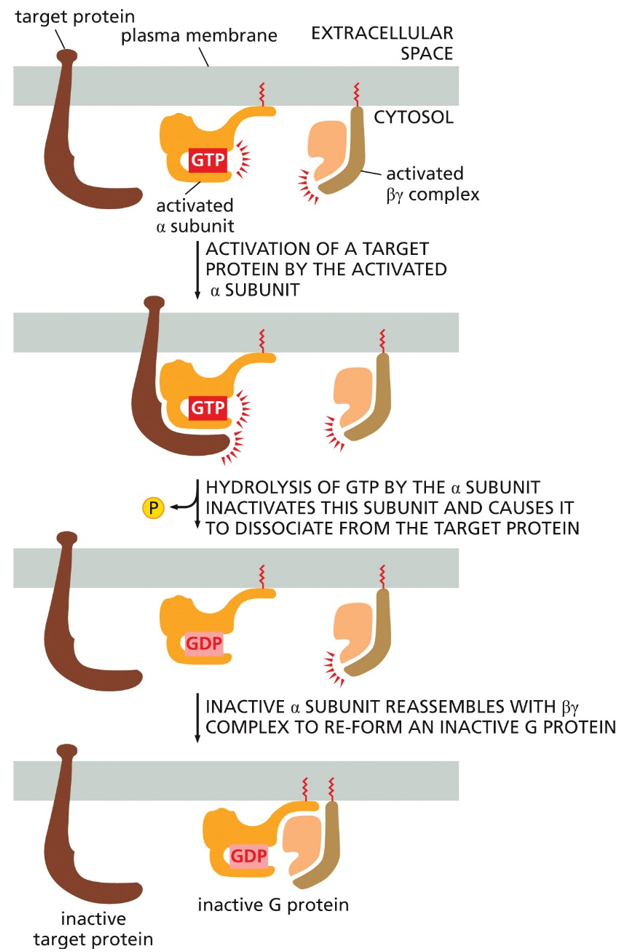
To inactivate it, the active alpha subunit will be targeted by the target protein, which is activated when bound by the alpha subunit. After time, the GTP will hydrolyze into GDP in the alpha subunit, and inactivate it, causing the target protein to dissociate. This inactive alpha subunit can then reassemble with beta and gamma to form the inactive g-protein again
The overall cycling of the G-protein has 4 steps.
1. Activate: GTP in exchange for GDP, g-protein activates2. Signal: Dissociation of the alpha subunit bound by GTP, leaves gamma and beta subunits
3. Inactivate: Hydrolysis of the alpha subunit’s GTP into GDP, inactivating it
4. Reset: The alpha subunit with GDP reassociates with beta gamma dimer to reform the inactive G-protein
Ach will bind to a G-protein (activating the alpha subunit). Here, the beta gamma dimer binds to a closed K+ potassium channel, allowing it to open and release K+ into the extracellular space. Once GTP is hydrolyzed, its affinity will cause it to bind to the beta gamma dimer and the K+ channel will close
Secondary messengers are short lived small intracellular signaling molecules that can be synthesized/released and broken down again in specific reactions by enzymes/ion channels. Elevated concentrations can lead to a rapid cellular response. Termination or degradation can lead to the stop of a cellular response. Some, such as Ca2+ can be stored and control released when needed. They can be localized, enabling the cell to limit space and time of signal activity.
Classes of secondary messengers include cyclic nucleotides like cAMP and cGMP, membrane lipid derivatives (DAG/IP3), Ca2+, and gasses (NO/CO)
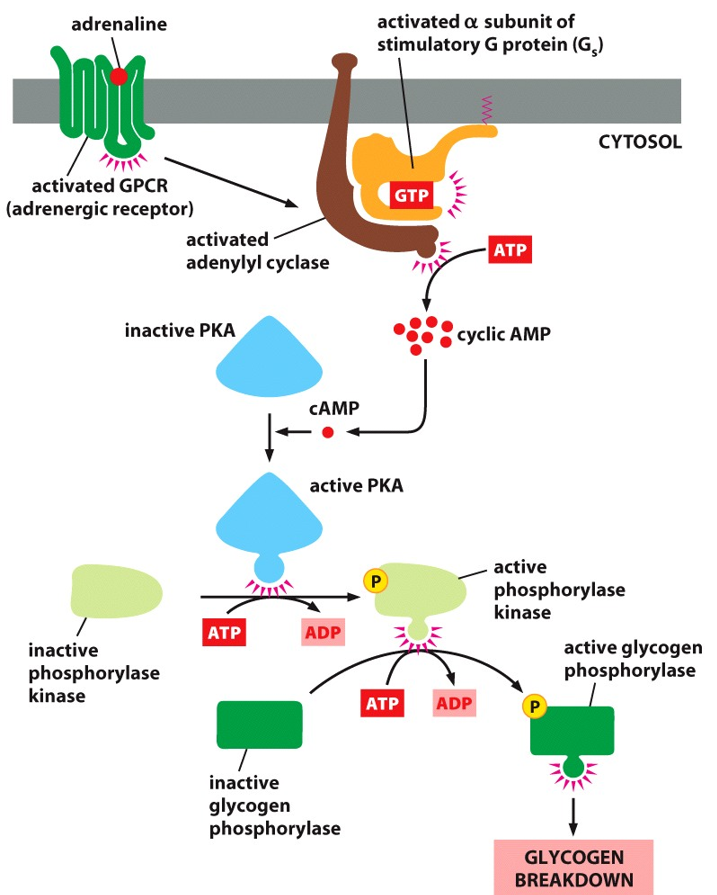
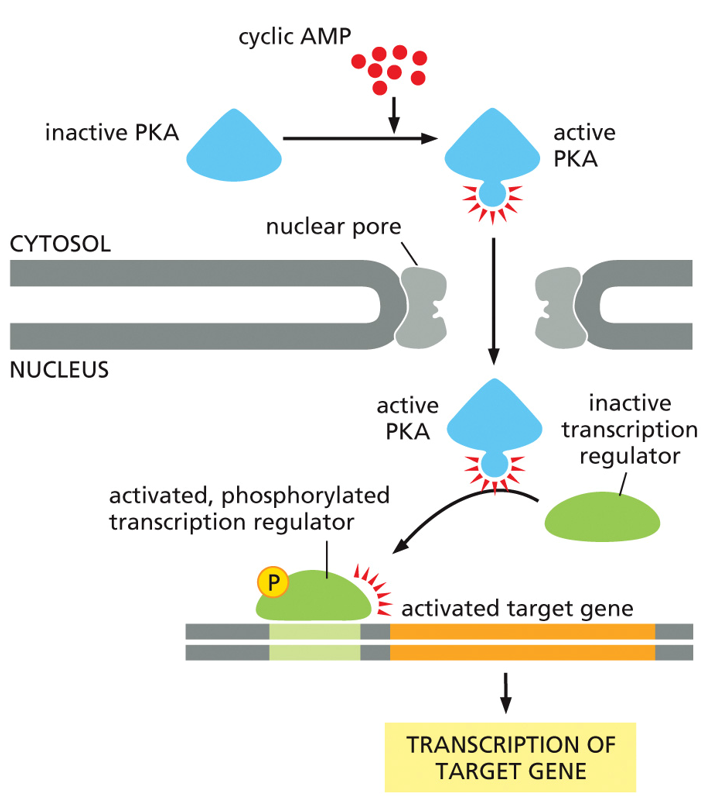
Cyclic AMP (cAMP) mediates other cellular responses. Adrenaline stimulation of glycogen breakdown involves the activation of Protein Kinase A (PKA). Adrenaline also helps stimulate gene transcription. PKA is a master kinase, meaning it can influence many other pathways in the cell. It has a profound effect. The trimeric G-protein is activated by adrenalin, which activates the alpha subunit which then activates adenylyl cyclase, which creates cAMP. Here, the cAMP activates PKA. PKA acts as a kinase to a phosphorylase kinase, activating glycogen phosphorylase, promoting glycogen breakdown into glucose molecules. In another pathway, PKA will diffuse into the nucleus and act as a kinase on transcription factors. First case leads to quicker responses, while gene regulation is slower, showing PKA’s diverse abilities.
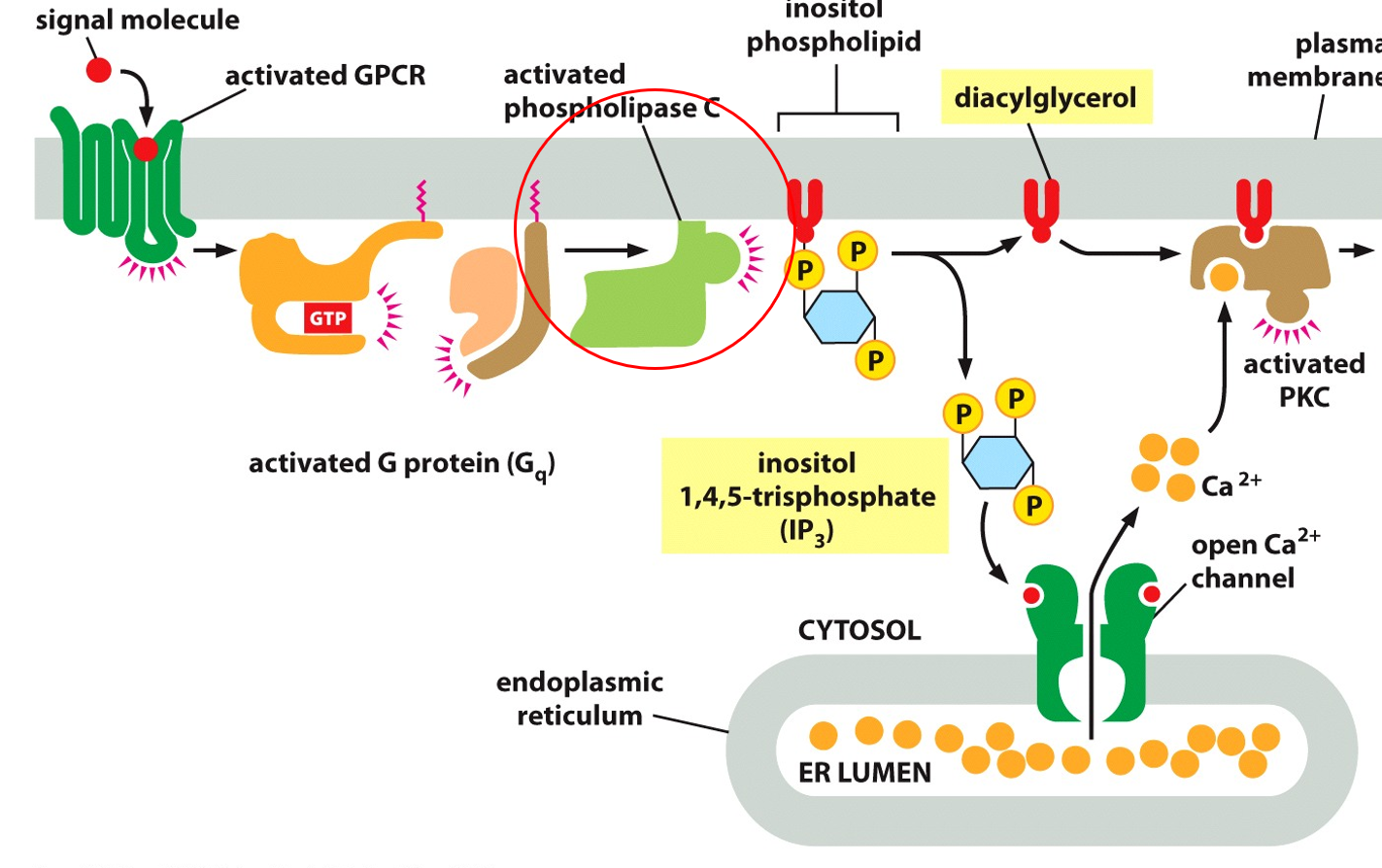
IP3 signaling cascades will activate protein kinase C (PKC). The GPCR is activated which activates the trimeric G-protein, alpha subunit activates PKC, which helps the break down of inositol phospholipid into diacylglycerol and inositol 1,4,5-trisphosphate (IP3). Both of these molecules are used in secondary messaging, diacylglycerol is a binding site for PKC, with the help of Ca2+ it can open ion channels. IP3 releases calcium ions when bound to receptors in the ER, and then with diacylglycerol allows for PKC to be activated. IP3 binds to a ligand gated channel to allow out Ca2+, which is used to bind to PKC to activate it, which only has a binding site for Ca2+ when bound to the diacylglycerol
Calmodulin is a calcium modulated protein that binds to Ca2+, and plays roles in inflammation, metabolism, apoptosis, smooth muscle contraction, intracellular movement, short-term/long-term memory, nerve cell growth, and the immune response. They are regulating proteins, that themselves are regulated by calcium.
Caffeine can act as both a ligand for receptors/channels, as well as act intracellularly on calcium and cAMP. It is a competitive inhibitor, binding to adenosine receptors (GPCRs) but not activating it. Less adenosine increases dopamine levels. Caffeine also impacts adrenaline levels positively.
Signal transduction pathways commonly involve the phosphorylation of amino acids
For an enzyme coupled receptor, it is inactive in its single helix form, and must be dimerized to be activated. The ligand will bind and bring subunits together, causing a conformational change to open up an allosteric binding site
Serine, threonine, and tyrosine are the 3 amino acids that can be phosphorylated (being the part of the protein that is holding the phosphate for a kinase/phosphorylase). Ones with a lot of tyrosine are RTKs.
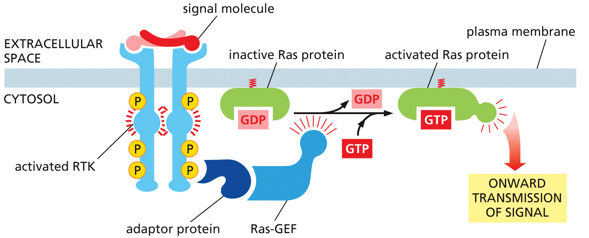
RTKs activate monomeric G-protein Ras. RAS is a superfamily of small G-protein. They are analogous to the alpha subunit, able to hydrolyze their own GTP into GDP to be inactive.
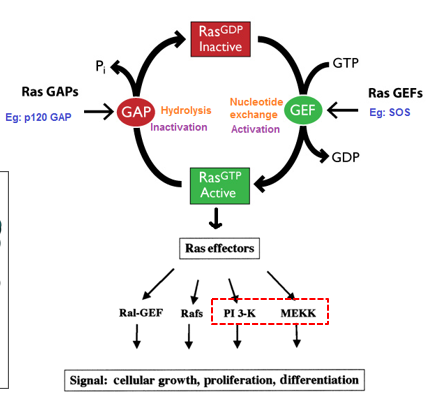
RAS are a superfamily of small g-protein, with only one small subunit. They tend to regulate cell proliferation and differentiation, along with gene expression, nucleocytoplasmic transport, cytoskeleton organization, and vesicle trafficking. They are master kinases. GEF is a GTP exchange factor, that increases the rate GDP is replaced by GTP (it itself isn’t doing the process just speeding it up). A GTPase activating protein (GAP) will increase the rate at which Ras hydrolyzes GTP into GDP. GEF and GAP help regulate GTP proteins
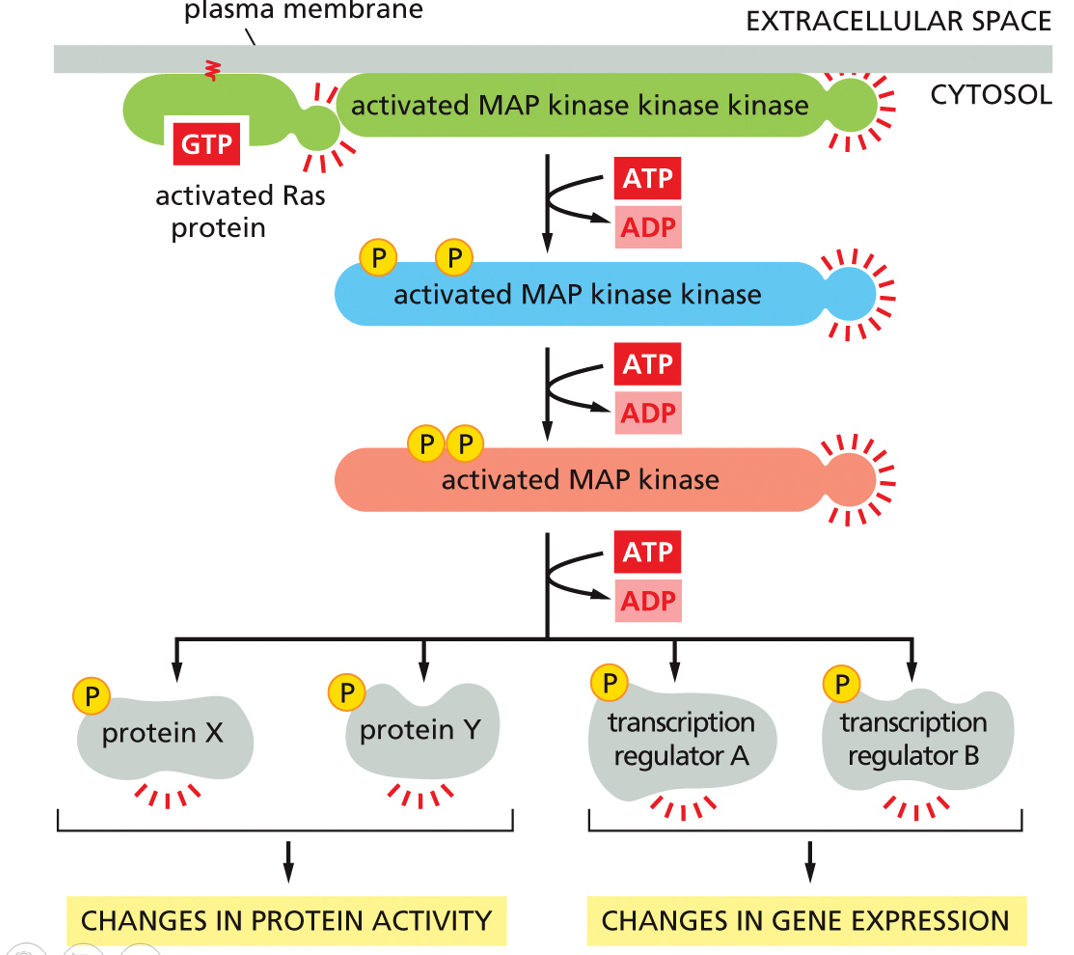
Along the plasma membrane, the activated Ras protein will signal an activated MAP kinase kinase kinase, and use 3 ATPs to phosphorylate various proteins and transcription regulators, which will change protein activity and gen expression. The MAP kinase is activated by the MAP kinase kinase, which is activated by the MAP kinase kinase kinase. This is the MAP kinase signaling pathway, and can have slow and fast responses
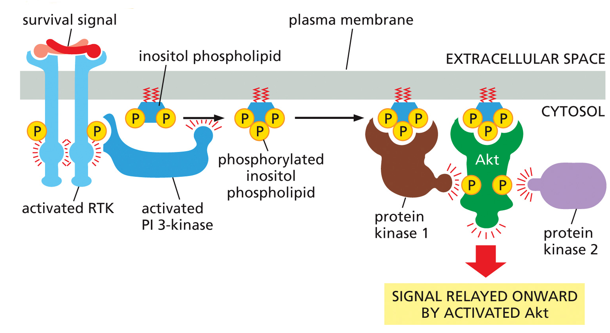
IP3 is also used in other pathways, but in order to stop it from becoming diacyl glycerol, it will be phosphorylated by a kinase. With the extra phosphate, it serves as a binding site for Akt, which serves as a binding site for 2 protein kinases
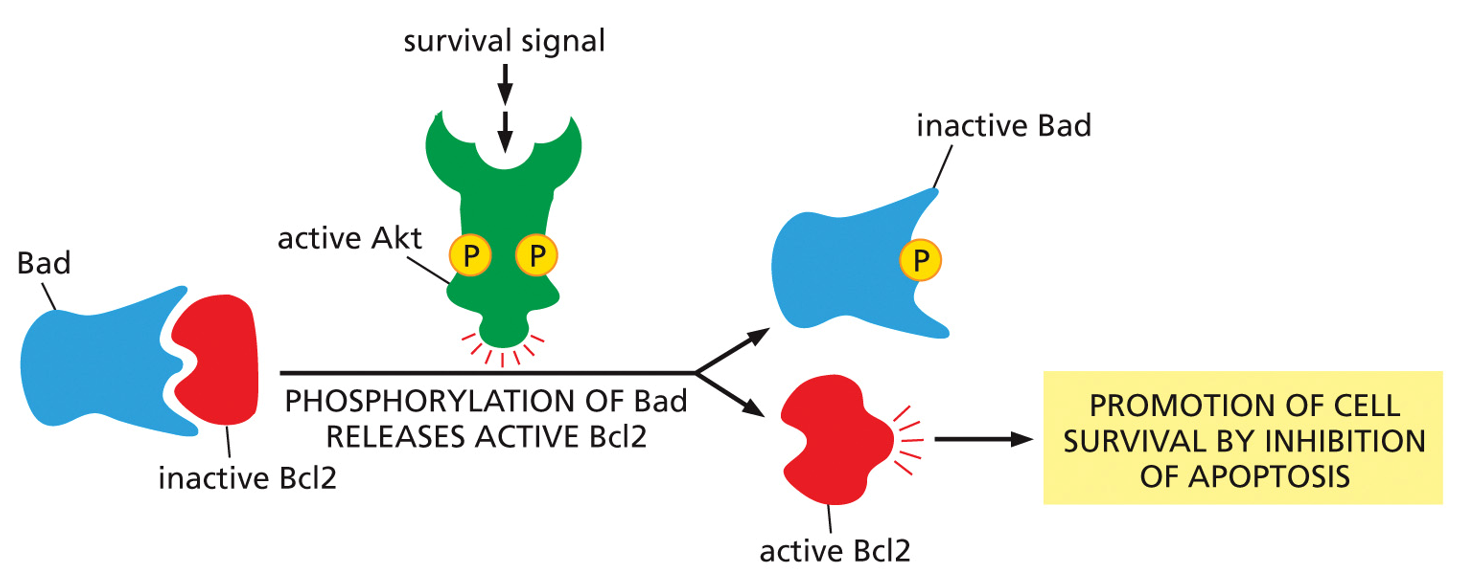
Apoptosis is prevented by signaling. Bcl2 is an apoptosis inhibitor, Bad wants to bind to it, and Bad is an apoptosis inhibitor. As long as Akt is simulated, the cell will survive
Activated Akt will also stimulate cell growth. Growth factors bind on the outside of the plasma membrane and activate RTK inside the cell, which activates P-3-kinase, which then activates Akt to activate Tor. Tor inhibits protein degradation and stimulates protein synthesis, both which lead to overall cell growth
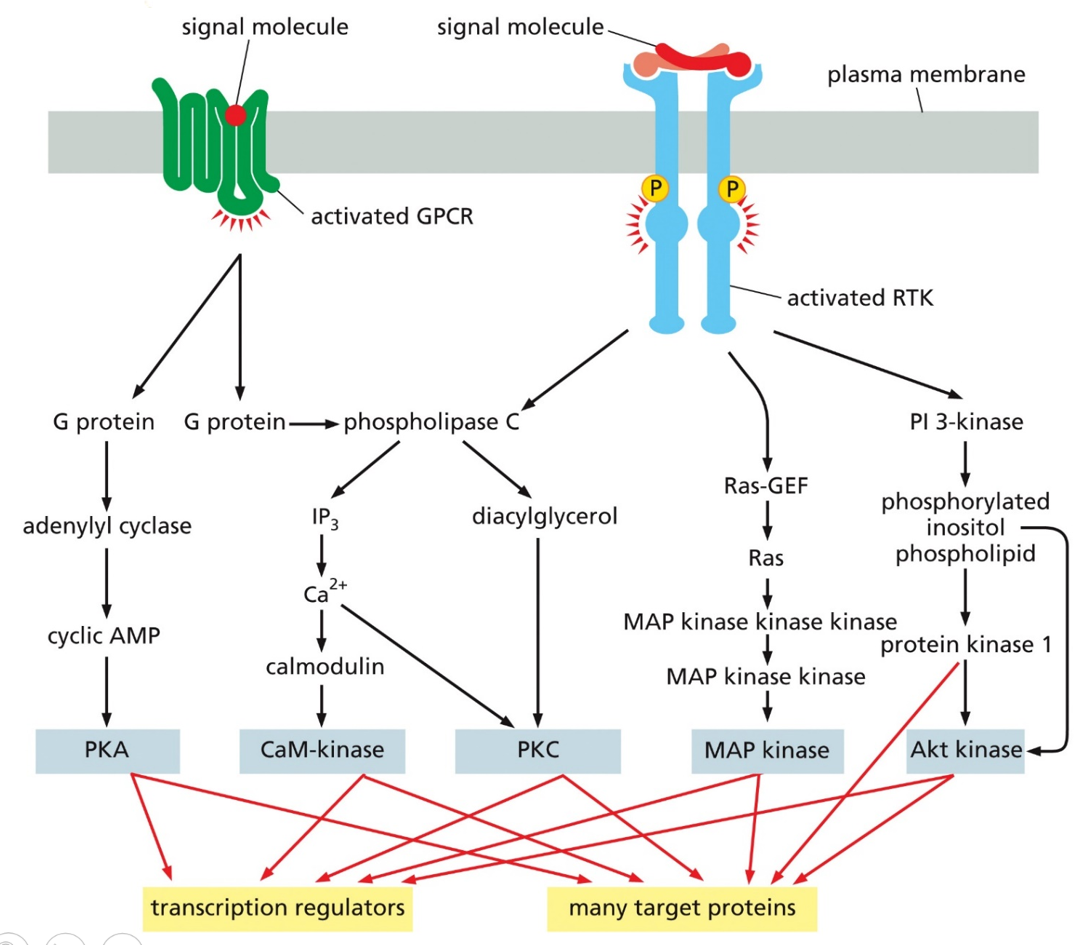
Many signaling pathways interlink with one another. He loves this image, memorize and understand these pathways
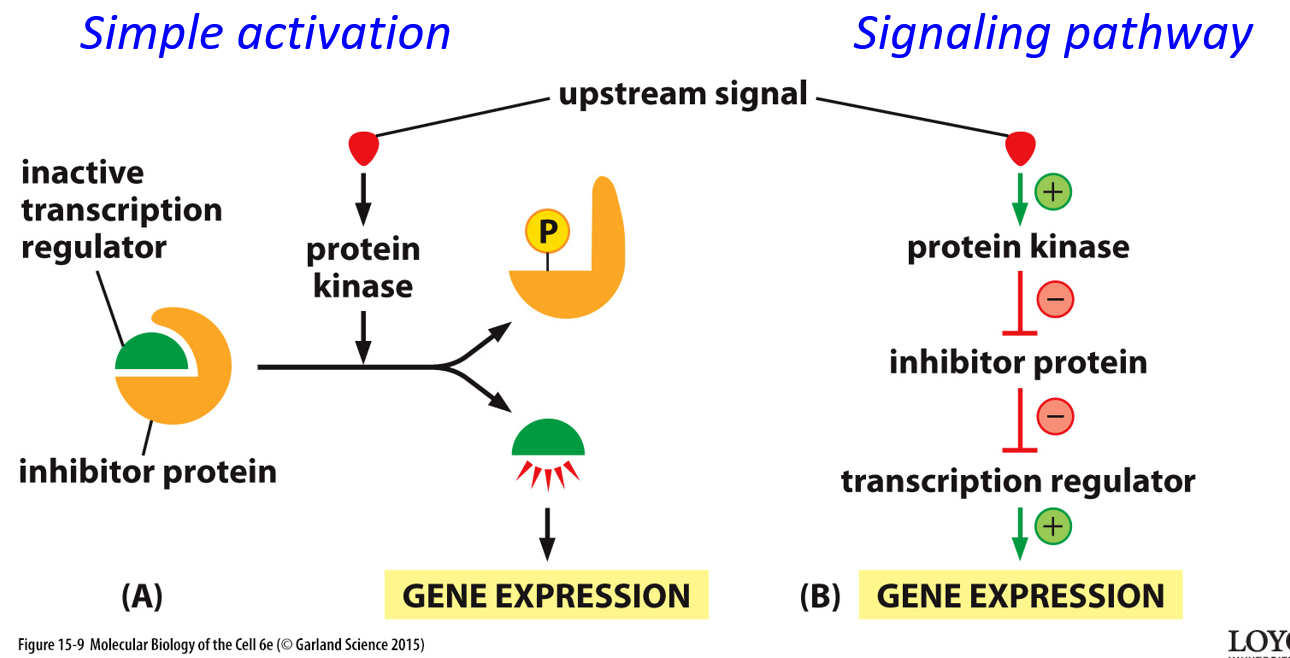
An upstream signal may go through simple activation, directly activating a protein, or it could go through a signal pathway, with inhibitors and transcriptional regulators. Negative signals always overpower positive ones.
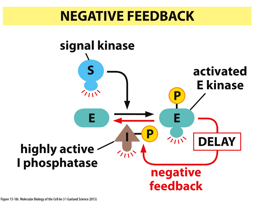
With negative feedback, the product of a pathway will also work to prevent the pathway from happening more, regulating a certain level of something. A product of the pathway can activate/deactivate various parts of the pathway
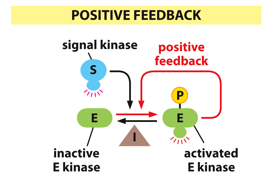
With positive feedback, an extreme is pushed further in one direction. A product can activate further parts of the pathway, creating more and more.
Signal integration describes how some target proteins require multiple signals in order to activate. These can be controlled by multiple different pathways, allowing for certain complex conditions to activate specific responses