3.5 Atomic Structure & The Periodic Table
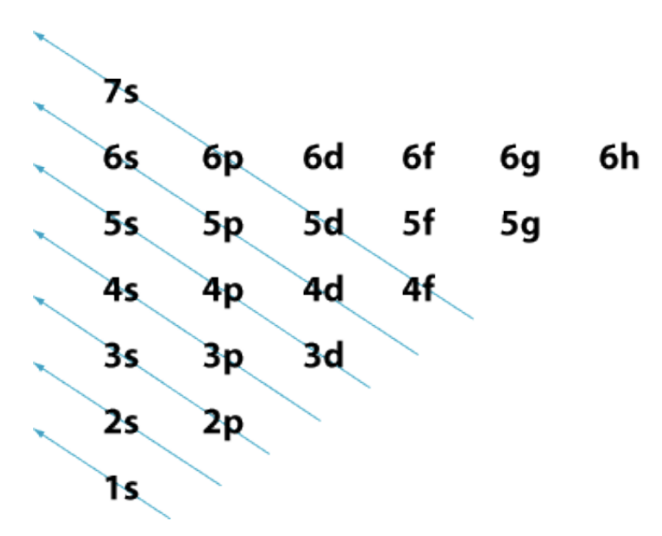
Aufbau Diagram
This is the order in which electrons fill the different orbitals
Recall that orbitals may overlap, which means you can fill a 4s before a 3d orbital
Electron Configuration
Determine the position of the element in the periodic table and the total number of electrons in the atom or ion.
- For anions: add extra electrons to equal to the charge
- For cations: subtract electrons to equal to the charge
Start assigning electrons in increasing order of main energy levels and sublevels using aufbau diagram or periodic table
Continue assigning electrons by filling each sublevel before moving to the next sublevel.
Abbreviated Electron Configuration
- use the previous noble gas + few extra electrons
- [Ar] 4s2
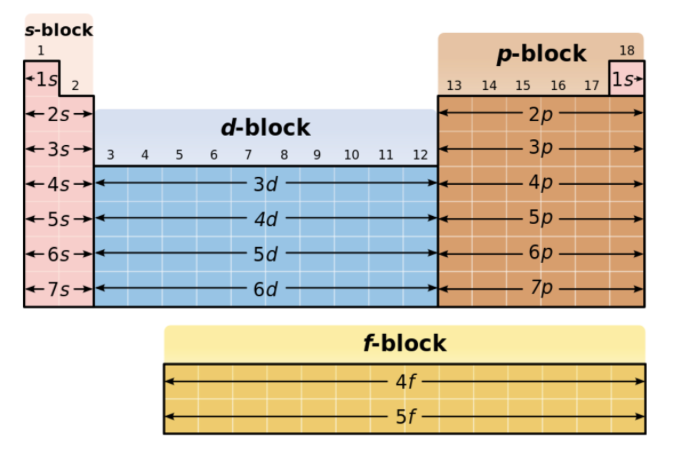
Periodic Table for Electron Configuration
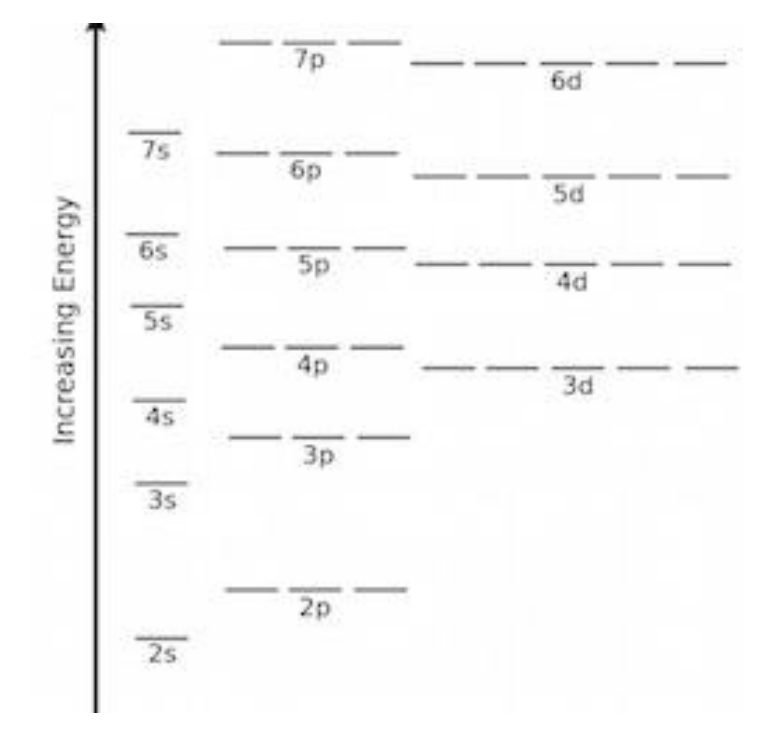
Energy Level Diagrams
Hund’s Rule: when several orbitals are at the same level of energy, one electron is placed into each orbitals before a second electron is added
Electrons in each level
- s = 2 electrons
- p = 6 electrons
- d = 10 electrons
- f = 14 electrons