Elements Review
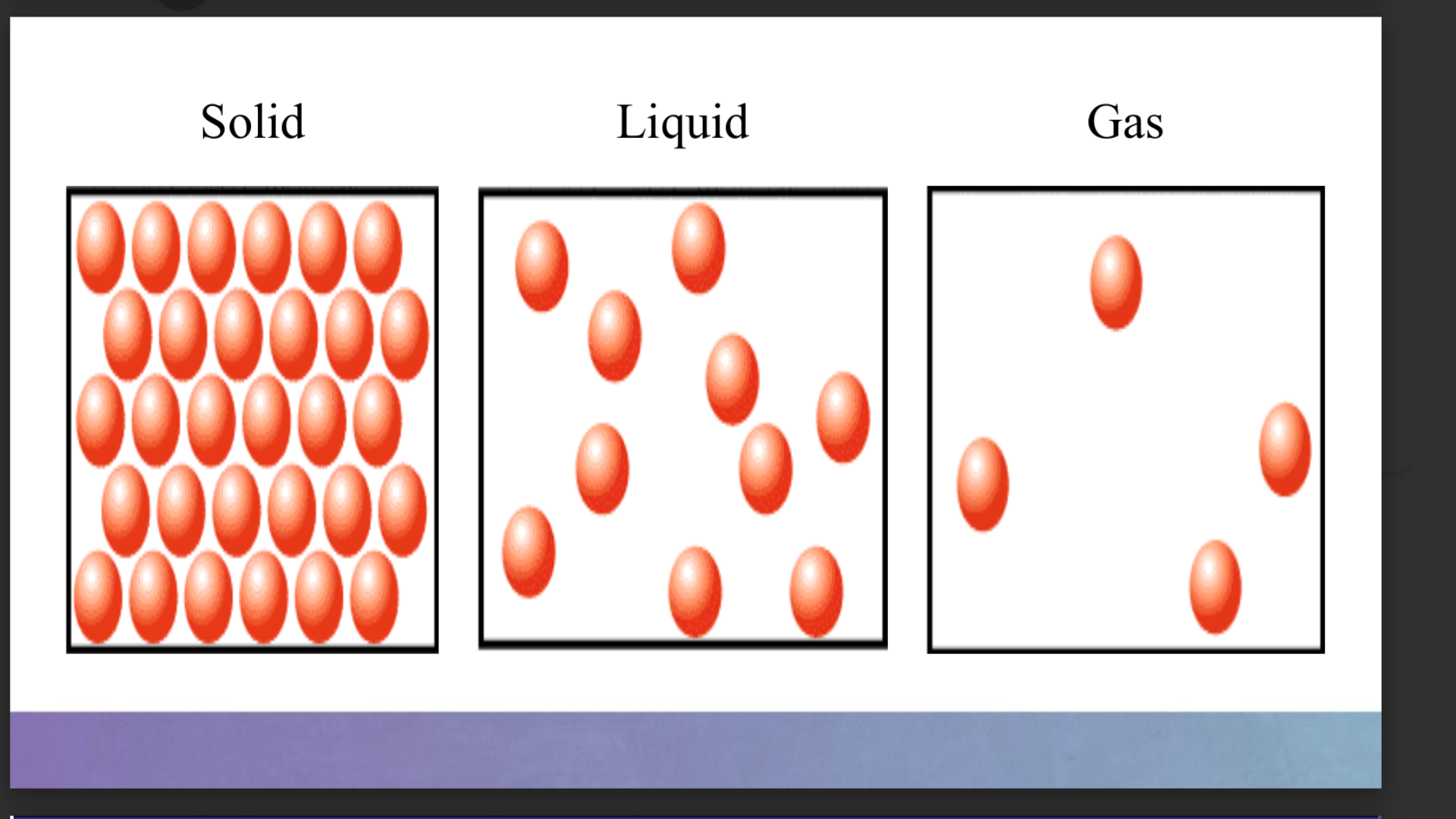
States of Matter
Solids
Have a definite shape and volume.
Particles are tightly packed and vibrate in fixed positions.
Examples: ice, rock, wood.
Liquids
Have a definite volume but take the shape of their container.
Particles are close together but can move around.
Examples: water, oil, milk.
Gases
Have no definite shape or volume; they expand to fill the available space.
Particles are widely dispersed and move randomly.
Examples: air, oxygen, steam.
Phase Changes
Melting
Solid to liquid (e.g., ice to water).
Occurs when the temperature increases and particles gain enough energy to break free from their fixed positions.
Freezing
Liquid to solid (e.g., water to ice).
Occurs when the temperature decreases and particles lose energy, forming a more ordered structure.
Boiling (or Vaporization)
Liquid to gas (e.g., water to steam).
Occurs when the temperature increases and particles gain enough energy to overcome intermolecular forces and escape into the gas phase.
Condensation
Gas to liquid (e.g., steam to water).
Occurs when the temperature decreases and particles lose energy, causing them to slow down and come closer together.
Sublimation
Solid directly to gas (e.g., dry ice to CO_2 gas).
Occurs when particles gain enough energy to bypass the liquid phase and directly enter the gas phase.
Deposition
Gas directly to solid (e.g., frost formation).
Occurs when particles lose energy and directly form a solid structure without passing through the liquid phase.
Key Properties
Density
Mass per unit volume (\rho = \frac{m}{V}, where \rho is density, m is mass, and V is volume).
Solids are generally denser than liquids, which are denser than gases, though there are exceptions.
Compressibility
Measure of how much the volume of a substance decreases under pressure.
Gases are highly compressible, liquids are slightly compressible, and solids are nearly incompressible.
Thermal Expansion
Tendency of matter to change in volume in response to changes in temperature.
Gases expand more than liquids, and liquids expand more than solids for the same temperature change.
Kinetic Molecular Theory
Basic Principles
Matter is composed of particles (atoms or molecules) in constant motion.
The kinetic energy of particles increases with temperature (higher temperature means faster movement).
Explains the behavior of gases, liquids, and solids based on the motion and arrangement of their particles.
Periodic Table
Atoms are the smallest unit of an element. They are made up of subatomic particles.
Protons are positive, and they mean the atomic number of an element.
Neutrons are neutral and are found by subtracting the atomic number from the atomic mass.
Electrons are negative, and they mean the atomic number of an element.
The formula for finding the maximum number of electrons that can fit in an electron shell is 2n², where 'n' represents the principal quantum number (energy level or shell number). For example, the first shell (n=1) can hold a maximum of 2 electrons, the second shell (n=2) can hold 8, and the third shell (n=3) can hold 18.
Here's a breakdown:
n = 1 (K-shell): 2(1)² = 2 electrons
n = 2 (L-shell): 2(2)² = 8 electrons
n = 3 (M-shell): 2(3)² = 18 electrons
n = 4 (N-shell): 2(4)² = 32 electrons
Metals, nonmetals, and metalloids are the three main classifications of elements based on their physical and chemical properties. Metals are typically shiny, malleable, and good conductors of heat and electricity. Nonmetals are generally brittle, poor conductors, and may exist as gases, liquids, or solids. Metalloids exhibit properties intermediate between metals and nonmetals, often appearing shiny but being brittle and having moderate conductivity.
Metals:
Physical Properties: Shiny (lustrous), malleable (can be hammered into thin sheets), ductile (can be drawn into wires), good conductors of heat and electricity, generally solid at room temperature (except mercury).
Chemical Properties: Tend to lose electrons and form positive ions.
Examples: Iron, copper, gold, aluminum.
Nonmetals:
Physical Properties: Brittle (easily broken), poor conductors of heat and electricity, can be gases, liquids, or solids at room temperature.
Chemical Properties: Tend to gain electrons and form negative ions.
Examples: Oxygen, hydrogen, nitrogen, chlorine, sulfur.
Metalloids:
Physical Properties: Appear shiny like metals but are often brittle like nonmetals, exhibit intermediate electrical conductivity (semiconductors).
Chemical Properties: Can behave as both metals and nonmetals depending on the reaction conditions.
Examples: Boron, silicon, germanium, arsenic, antimony, tellurium, polonium.
In the periodic table, periods are the horizontal rows, while groups (also called families) are the vertical columns. Elements within the same period have the same number of electron shells, and elements within the same group have similar chemical properties.
Key points about periods:
Periods are numbered 1 through 7 from top to bottom.
Each period represents a new electron shell being filled.
Elements in the same period do not necessarily have similar properties, though some trends can be observed across a period.
Key points about groups:
Groups are also numbered 1 through 18, although older systems sometimes use Roman numerals.
Elements within the same group tend to have similar chemical properties because they have the same number of valence electrons.
For example, the alkali metals (Group 1) all have one valence electron and react similarly with water.
One important thing about the periodic table is that it each period and group have specific characteristics.
Valence Electrons
Valence electrons are the electrons located in the outermost shell or energy level of an atom, which are responsible for the atom's chemical properties and participate in the formation of chemical bonds. These electrons determine how an atom reacts with other atoms and the types of bonds it can form.
Definition and Role of Valence Electrons
Valence electrons are specifically the electrons in the outermost electron shell of an atom, making them the least tightly held by the nucleus. This allows them to interact readily with other atoms during chemical reactions. In the context of covalent bonding, each atom contributes one valence electron to form a shared pair, which constitutes the bond.
Chemical Properties Determined by Valence Electrons
The presence and number of valence electrons dictate an element's reactivity, electronegativity, and bonding capacity. Atoms with a full outer shell of valence electrons tend to be chemically inert, such as noble gases with a stable octet configuration. Atoms with one or two electrons more or fewer than a full shell are typically highly reactive because of their tendency to lose, gain, or share electrons to achieve stability.
Valence Electrons in Different Types of Elements
- For main-group elements, valence electrons reside exclusively in the outermost shell (highest principal quantum number).
- For transition metals and inner transition metals, valence electrons can also be in the (n-1)d or (n-2)f subshells, which are close in energy to the outer shell, and these sublevel electrons also participate in bonding.
Determining the Number of Valence Electrons
The number of valence electrons corresponds with the element’s group (column) in the periodic table. For groups 1–2 and 13–18, the number of valence electrons is usually indicated by the group number or its units digit, with certain exceptions like helium. Transition metals are an exception and require specific examination of their electron configurations.
Importance in Bond Formation
Valence electrons are the primary actors in chemical bonding, either being shared in covalent bonds or transferred in ionic bonds. They influence the physical properties such as electrical conductivity and the chemical behavior of elements.
In summary, valence electrons are the chemically active electrons of an atom residing in its outer shell, essential for determining the atom's chemical behavior, bonding patterns, and reactivity.
Matter
Matter is broadly classified into two categories: pure substances and mixtures. Pure substances have a consistent composition and properties, like elements or compounds. Mixtures, on the other hand, are combinations of two or more pure substances, and can be homogeneous or heterogeneous.
Pure Substances:
Elements:
Basic building blocks of matter, composed of only one type of atom (e.g., oxygen, hydrogen).
Compounds:
Formed when two or more elements chemically combine in a fixed ratio (e.g., water (H₂O), salt (NaCl)).
Mixtures:
Homogeneous Mixtures:
Components are evenly distributed and appear uniform throughout the sample (e.g., saltwater, air).
Heterogeneous Mixtures:
Components are not evenly distributed and can be visually distinguished (e.g., dirt, gravel).
Acids and Bases
Acids
Sour taste
Sticky feel
turns litmus paper red
reacts with bases to form water and salt
0 - 6
Neutral
pH of 7.0 only
Bases
Bitter taste
slippery feel
turns litmus paper blue
reacts with acids to form water and a salt
8 - 14