Chapter 8: Basic Concepts of Chemical Bonding
Chemical Bonds
Three basic types of bonds, focus on the following two
Ionic (metal + nonmetal)
Electrostatic attraction between ions
Covalent (nonmetal + nonmetal)
Sharing of electrons
Metals donate electrons
Nonmetals accept electrons
Ionic Bonding
Metals and nonmetals (except group 8A)
Electron are transferred
Very exothermic (release of energy)
If an element has low ionization energy, it means the element gives up an electron
Another element readily gains an electron if is has a high electron affinity.
Octet rule: the element must have 8 electrons.
Example:
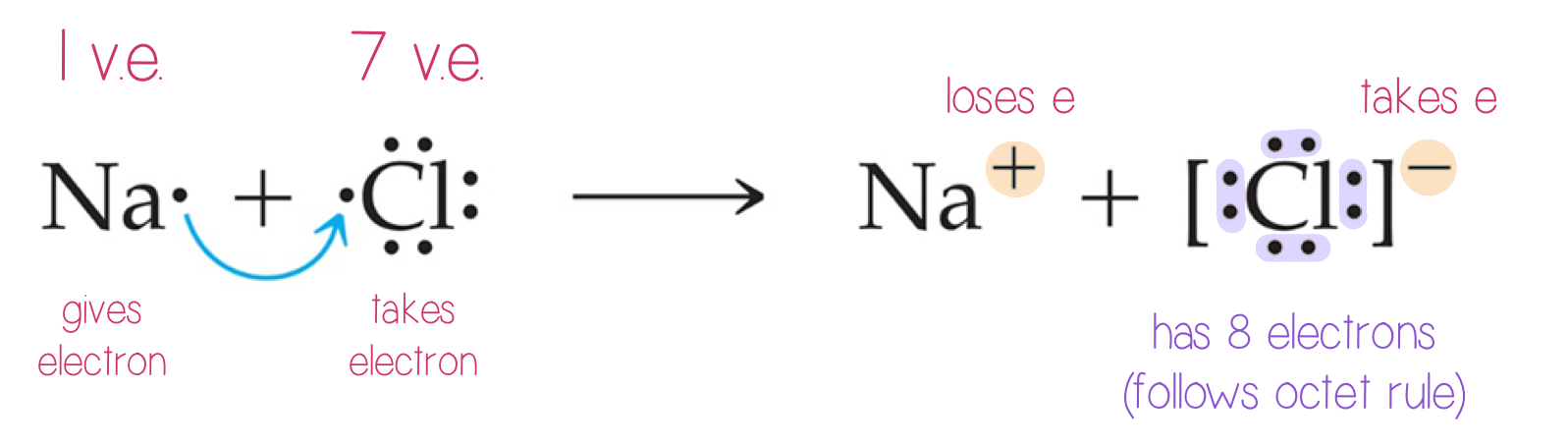
Covalent Bonding
Nonmetal and nonmetal
Electrons are shared
There are several electrostatic interactions in these bonds:
Attractions between electrons and nuclei
Repulsions between electrons
Repulsion between nuclei
For a bond to form, the attractions must be greater than the repulsions
Lewis Symbols
When forming compounds, atoms tend to gain, lose, or share electrons until they are surrounded by eight valence electrons (the octet rule).
Do not draw more than two electrons per side.
First, fill each side with a singular electron.
There will never be more than eight electrons.
Lewis Structures
Sharing electrons to make covalent bonds can be demonstrated using Lewis structures.
First, give each atom the same number of electrons as the nearest noble gas by sharing electrons.
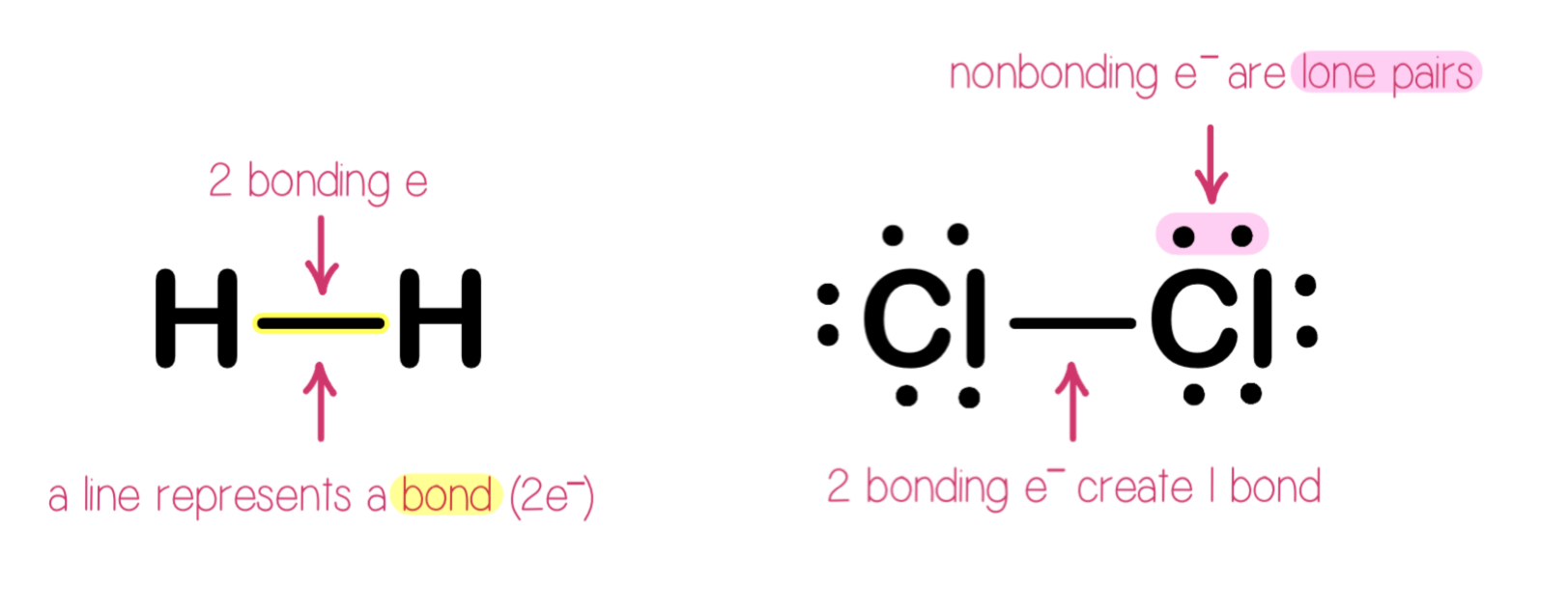
Electrons on Lewis Structures
Lone pairs: electrons located on only one atom in a Lewis structure.
Bonding pairs: shared electrons in a Lewis structure; they can be represented by two dots or one line, NOT BOTH!
Example
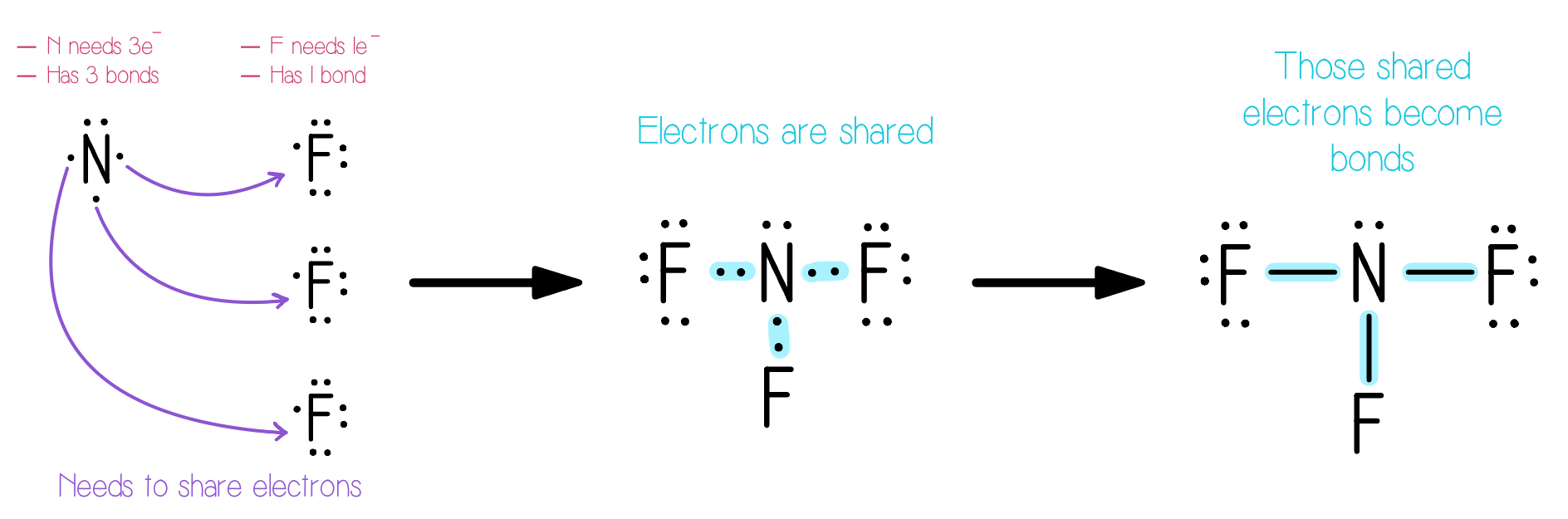
Given the Lewis symbols for nitrogen and fluorine, predict the formula of the stable. binary compound formed when nitrogen reacts with fluorine and draw its Lew structure.
How many total non-bonding electrons are there is Cl2? How many lone pairs?
12 non-bonding
6 lone pairs
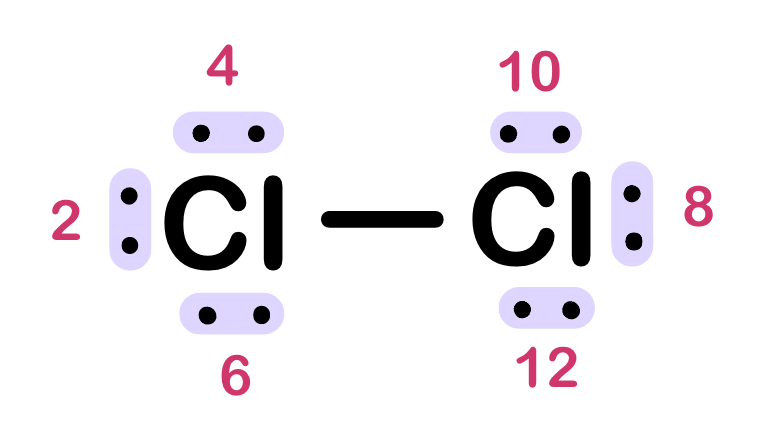
Examples for Hydrogen and Chlorine
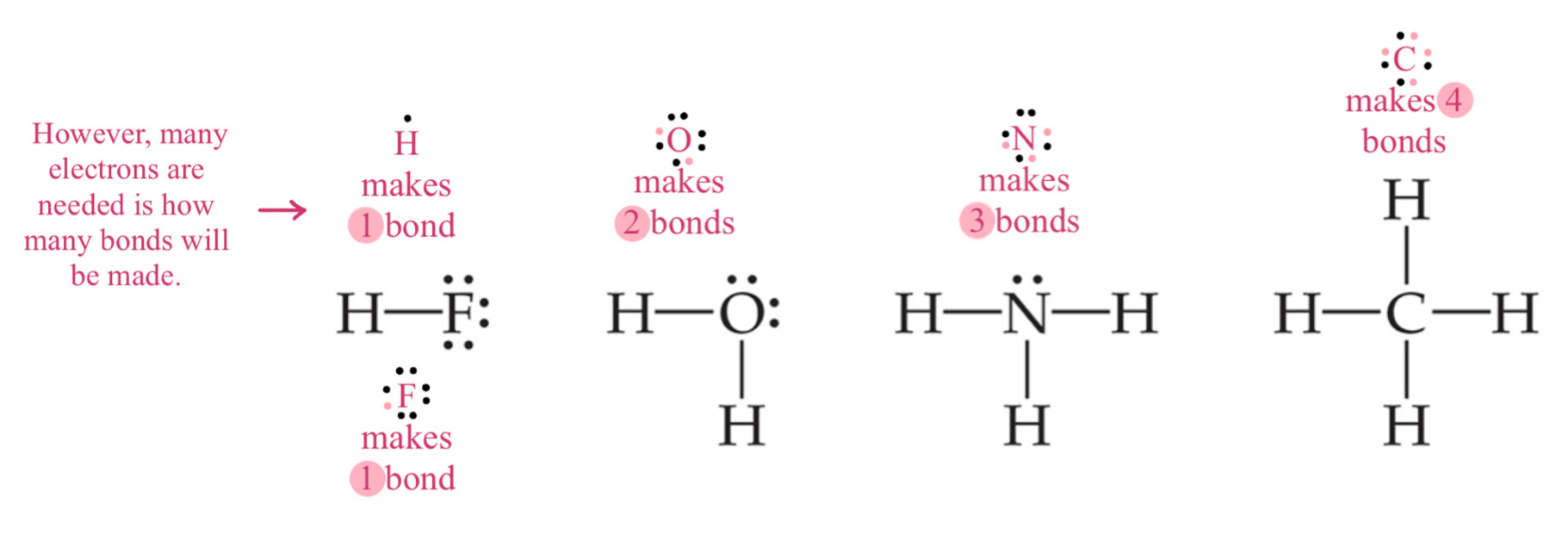
Number of Bonds for Nonmetals
The group number is the number of valence electrons.
To get an octet in simplest covalent molecules for nonmetals, the number of bonds needed will be the same as the electrons needs to complete the octet.
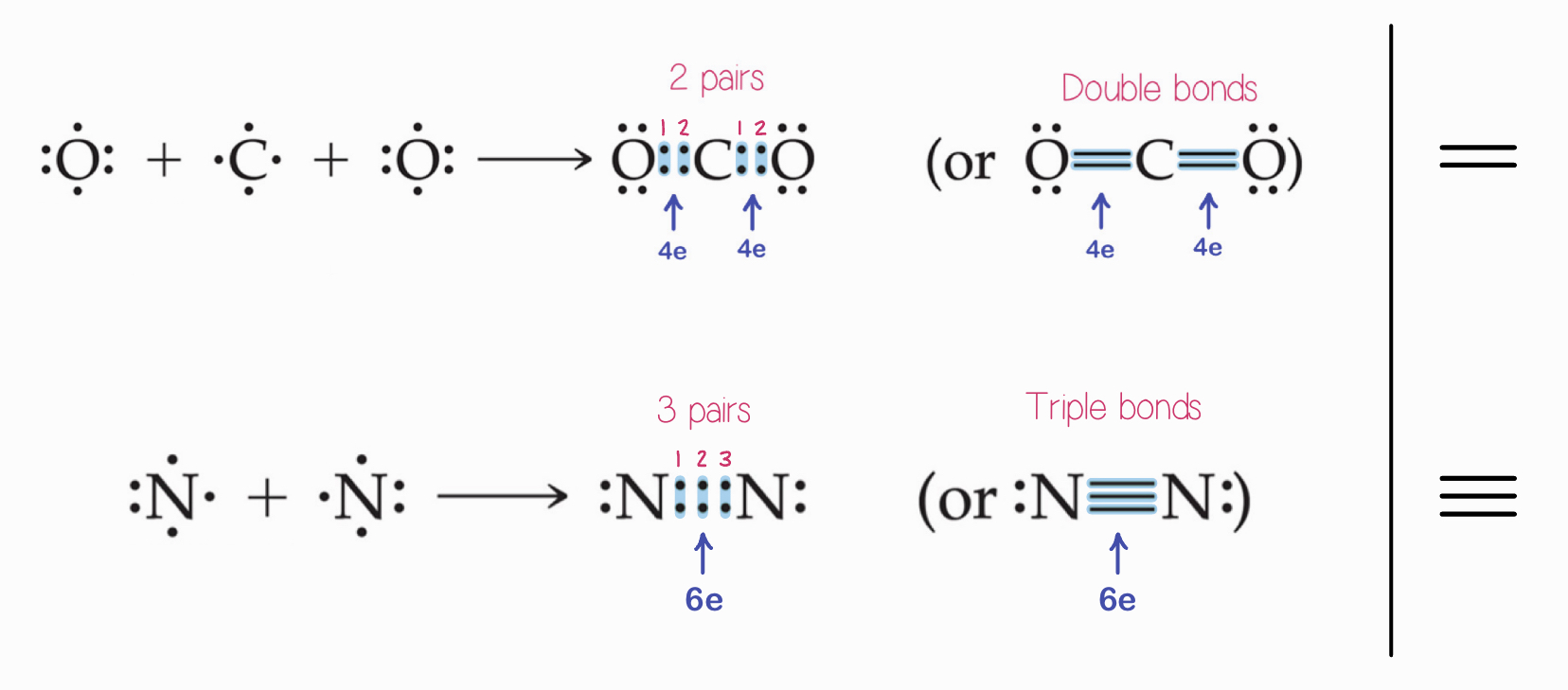
Multiple Bonds
Single bonds are formed when atoms share only one pair of electrons
1 lone pairs, 2e total, 1 bonds
Double bonds are formed when two pairs of electrons are shared (4e total).
2 lone pairs, 4e total, 2 bonds
Triple bonds are where three pairs of electrons are shared (6e total).
3 lone pairs, 6e total, 3 bonds
Polarity of Bonds
The electrons in a covalent bond are not always shared equally.
Bond polarity: a measure of how equally or unequally the electrons in a covalent bond are shared.
In a non-polar covalent bond, the electrons are shared equally.
In a polar covalent bond, one of the atoms attracts electrons to itself with a greater force.

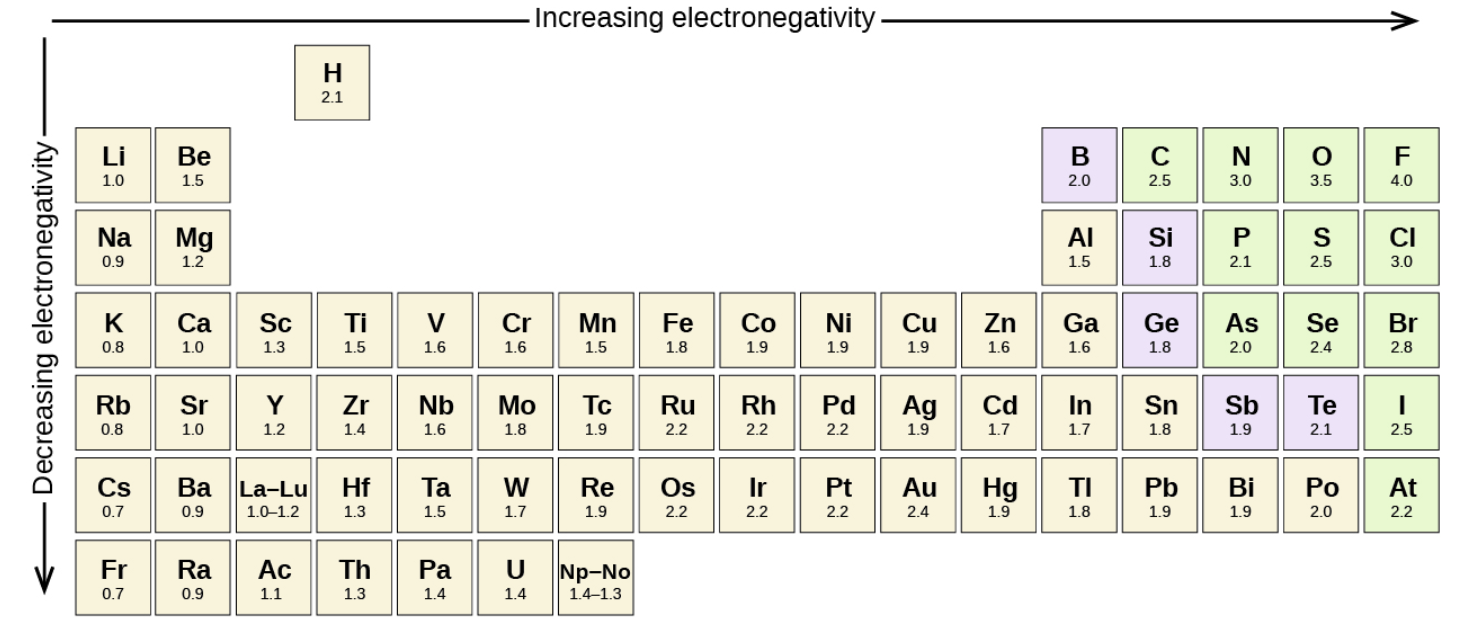
Electronegativity
Electronegativity: the ability of an atom in a molecule to attract electrons to itself.
F is the most electronegative
Fr is the least electronegative
On the periodic table, electronegativity…
Increases from left to right
Decreases from bottom to top
You subtract the higher electronegative value from the smaller one.
Electronegativity and Polar Covalent Bonds
When two atoms share electrons unequally, a polar covalent bond results.
Electrons stay around the more electronegative atom and the result is a partial negative charge.
Represented by \delta +
The other atom is more positive.
Represented by \delta -
Example:
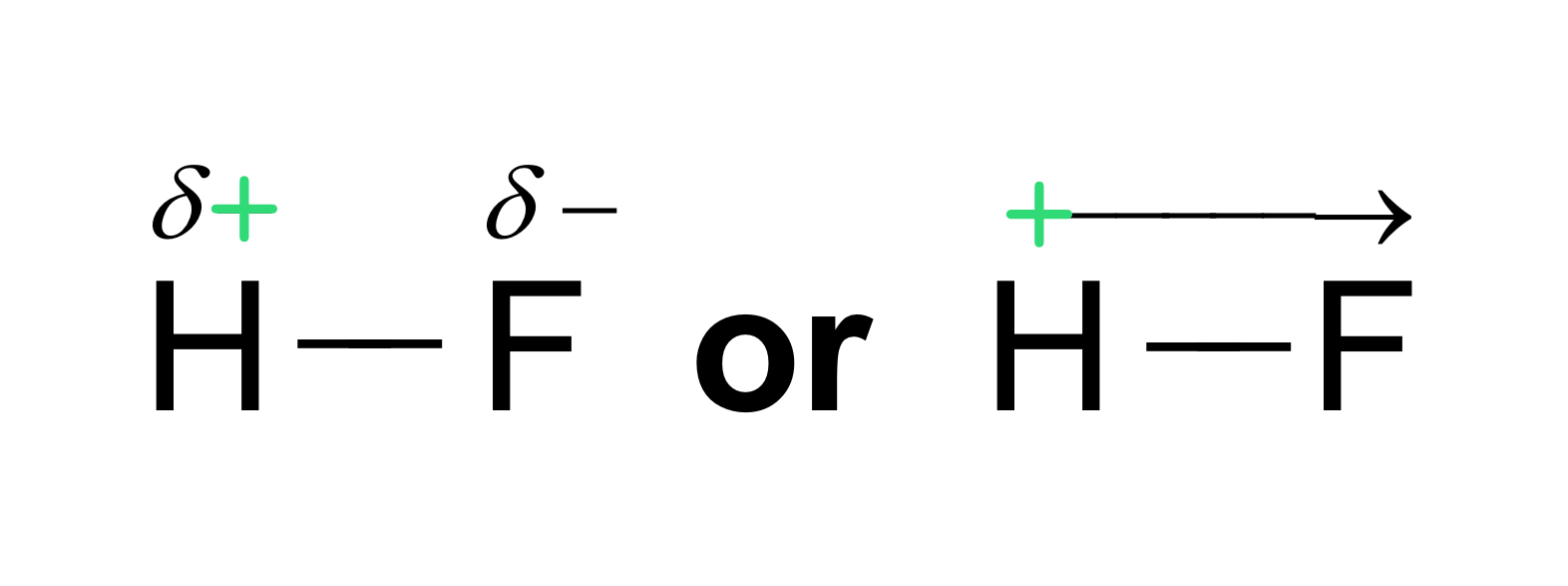
The greater the difference in electronegativity, the more polar the bond is.
H and F are very polar (unequal)
Example: Which bond in more polar (which has the greater electronegative difference)?

Dipoles
When two equal, but opposite, charges are separated by a distance, a dipole forms.
A dipole moment, \mu , produced by two equal but opposite charges separated by a distance, r, is calculated using the follow formula:
\mu=Qr
\mu is dipole moment (unit: debyes)
Q is charge (unit: Coulomb C)
r is distance (units: Angstrom \AA )
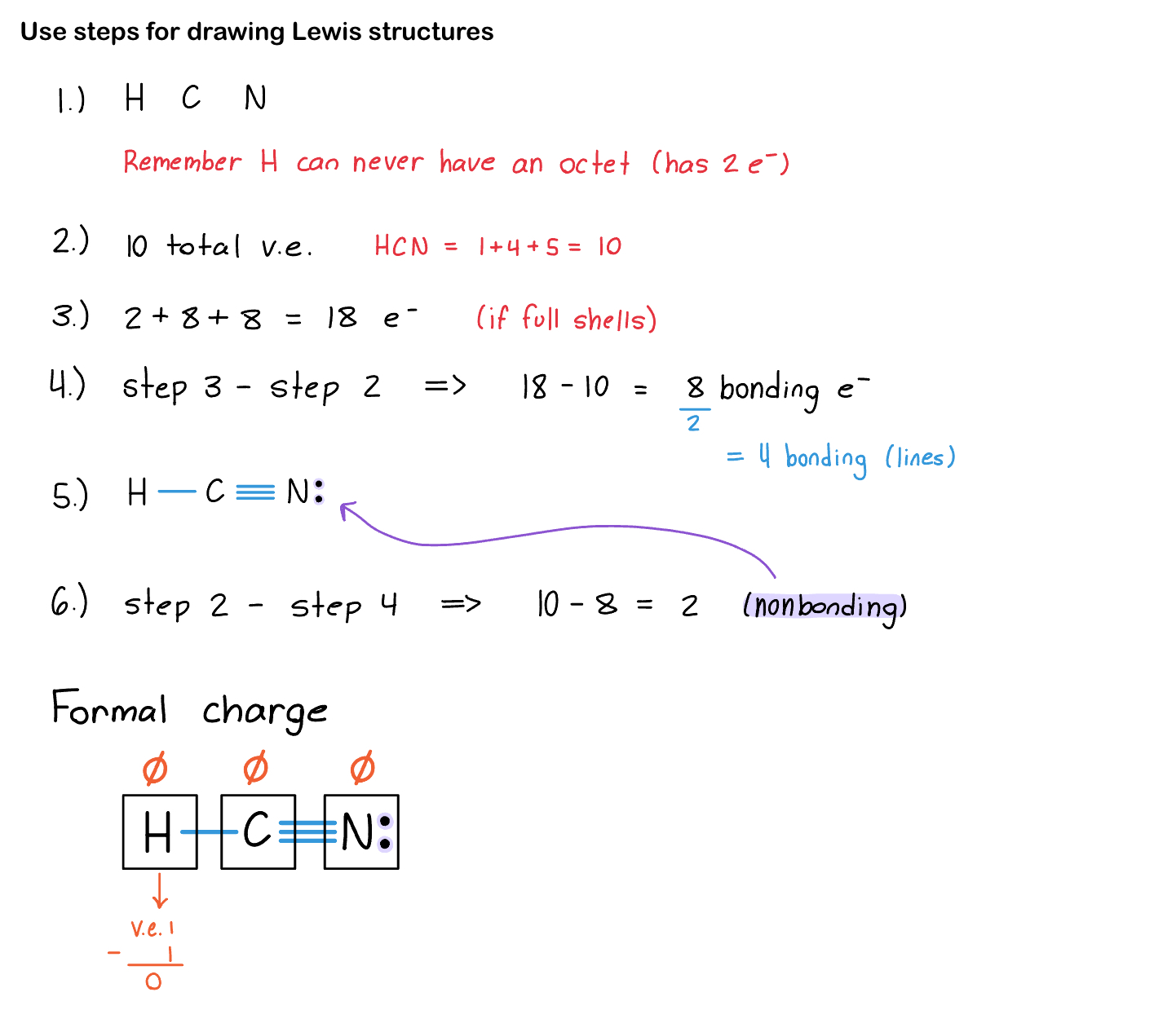
Steps for Drawing Lewis Structures
Arrange the atoms.
Count the total valence electrons.
Count the total number of electrons IF every single atom had an octet (or full valence shell). 8 for all atoms and 2 for hydrogen).
Subtract: Step 3 - Step 2 = # of bonding e
This # divided by 2 is how many bonds you’ll have. This includes single, double, and triple bonds.
Put in all the bonds calculated from step 4. Do single bonds first, and if you still have bonds left over, do double and triple bonds (for atoms that can handle double and triple bonds).
Now lone pairs (or non-bonding electrons):
Subtract: Step 2 - Step 4 = # of non-bonding e
Put those on the atoms that still don’t have an octet.
When you are done, check your work:
Did you use all the valence electrons?
Does every atoms have an octet? (except hydrogen)
Check formal charge.
Formal Charge
Formal charge is the charge an atom would have if all of the electrons in a covalent bond were shared equally.
Calculating formal charge:
(valence electrons) - \dfrac{1}{2} (bonding electrons) - (all nonbonding electrons)
FC = v.e. - lines - dots
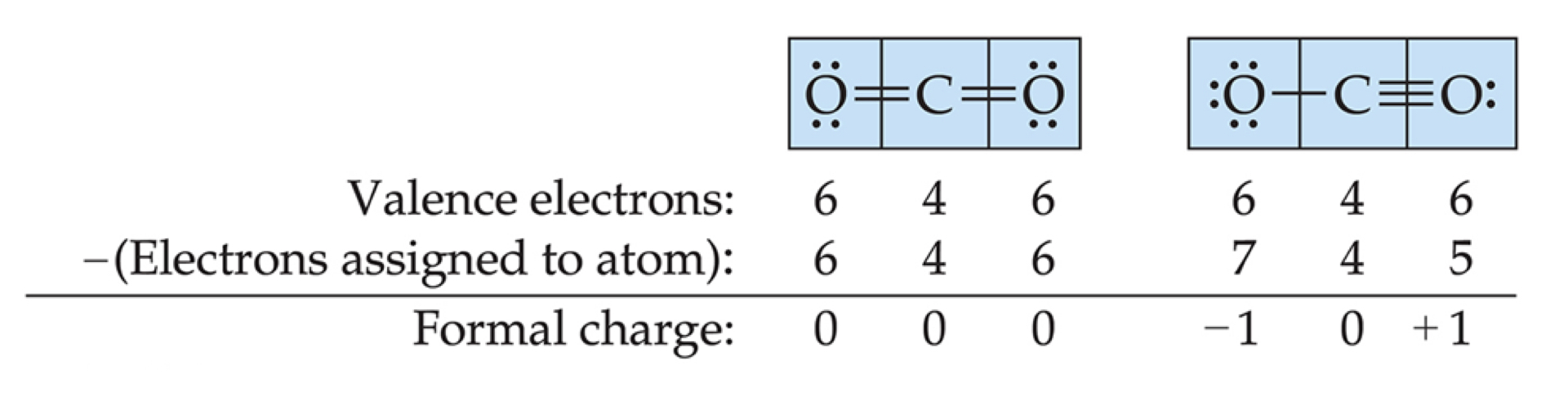
Lewis Structures With a Multiple Bond
If there is not enough electrons to give the central atom an octet, try multiple bonds.
Example:
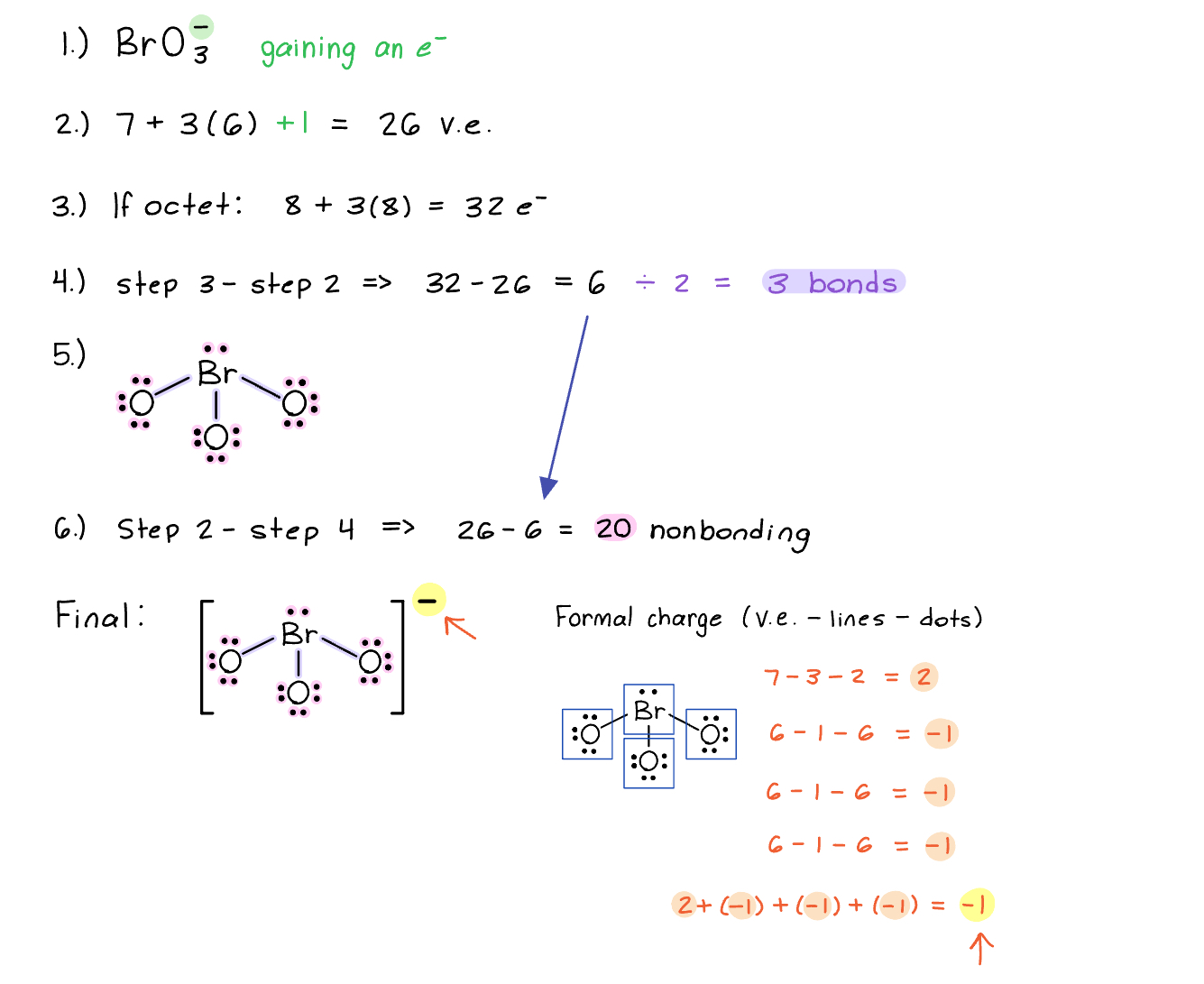
Lewis Structure for a Polyatomic Ion
The charge of a polyatomic results in the gain or loss of electrons.
Example:
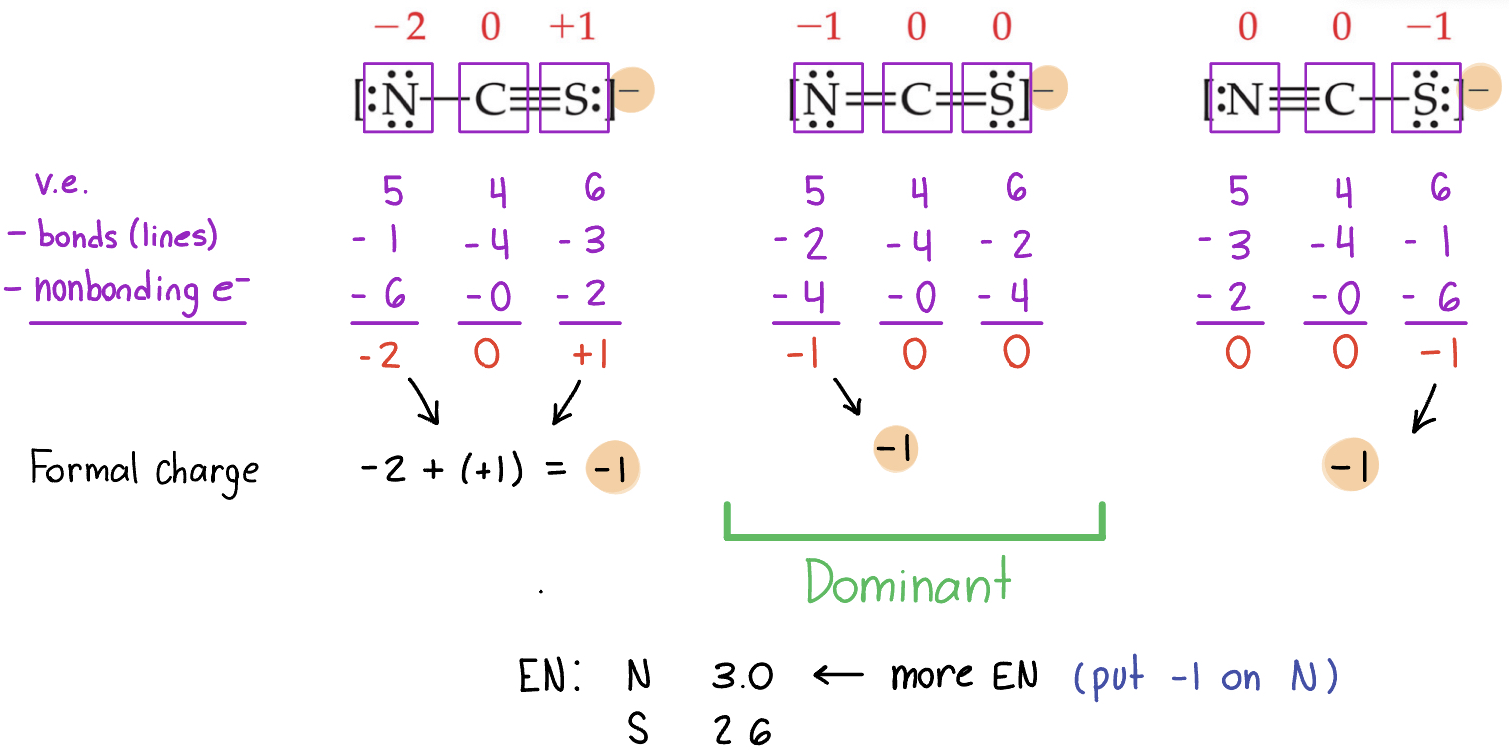
Writing Lewis Structures
The dominant Lewis structure…
is the one in which atoms have formal charges closest to zero.
puts a negative formal charge on the most electronegative atom.
F is more electronegative than Cl.
Example: There are three possible Lewis structures for the thiocyanate ion, NCS-. a.) Determine the formal charge in each structure and b.) based on the formal charge determine which Lewis structure is the dominant one.
Exceptions to the Octet Rule
There are three types of ions or molecules that do not follow the octet rule:
Ions or molecules with an odd number of electrons.
Ions or molecules with less than an octet.
Ions or molecules with more than eight valence electrons (expanded octet).
Odd Number of Electrons
Though rare and unstable, there are ions and molecules with an odd number of electrons.
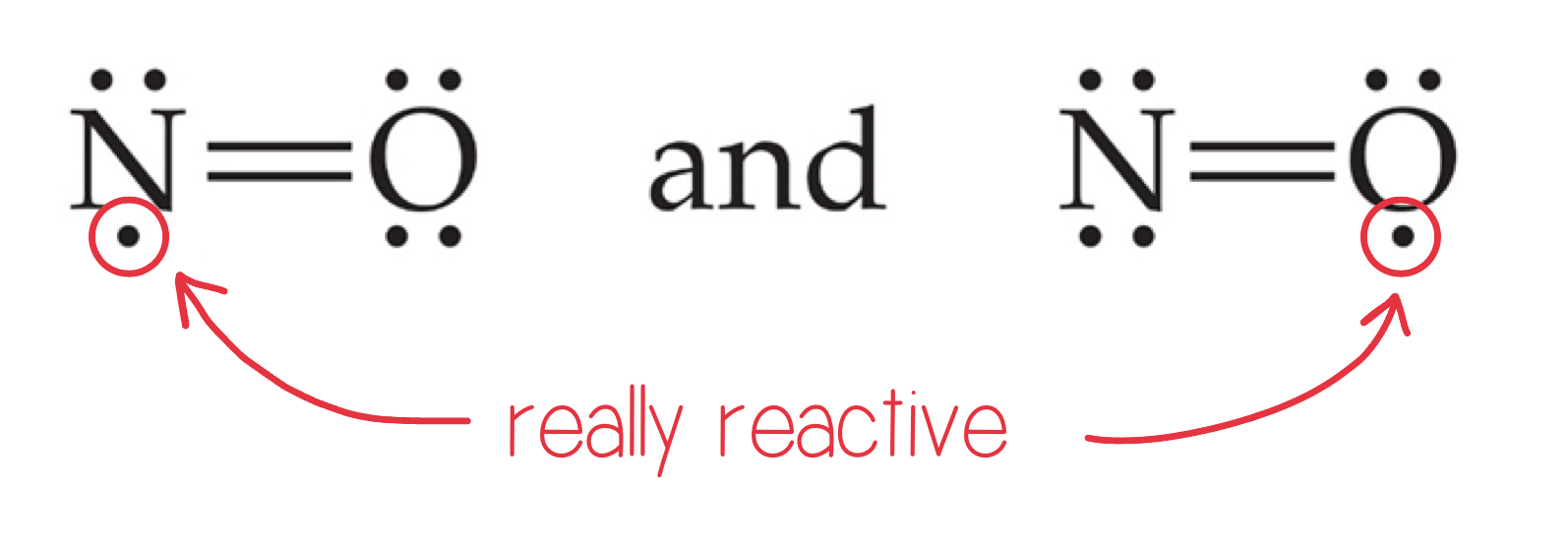
Fewer Than Eight Electrons
Elements in the second period before carbon (Li, Be, B, and also Al) can make stable compounds with fewer than eight electrons.
If filling the octet of the central atom results in a negative formal charge on the central atom and a positive formal charge on the more electronegative outer atom, don’t fill the octet of the central atom.
Example: Draw the Lewis structures for the following molecules
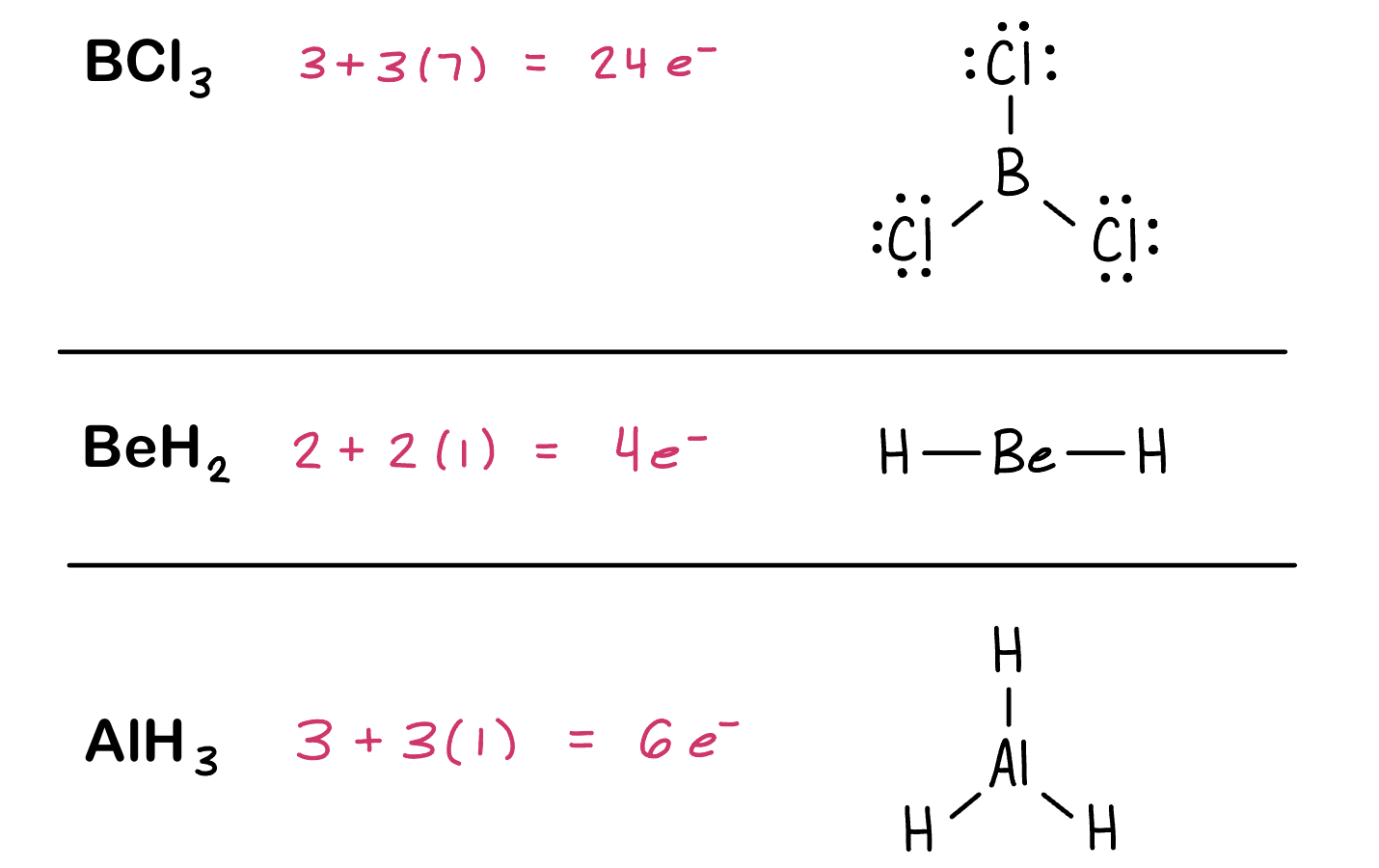
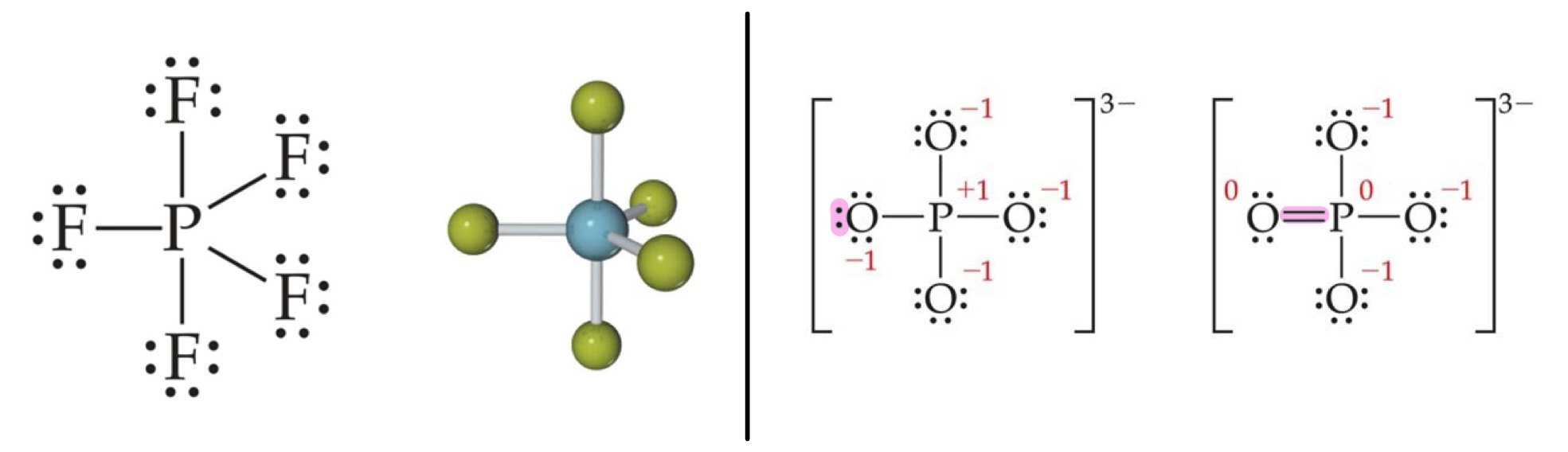
More Than Eight Electrons
When an element is in periods 3 through 6, it can use d-orbitals to make more than four bonds and be hypervalent.
Example: PF5 and PO_{4}^{3-} (Phosphate)
Phosphate will have four resonance structures with five bonds on the P atom)
Resonance (hybrid structure)
One Lewis structure cannot accurately depict a molecule like ozone.
Resonance is the movement of electrons.
We use multiple structures, resonance structures, to describe the molecule.
The organic compound benzene, C6H6, has two resonance structures.
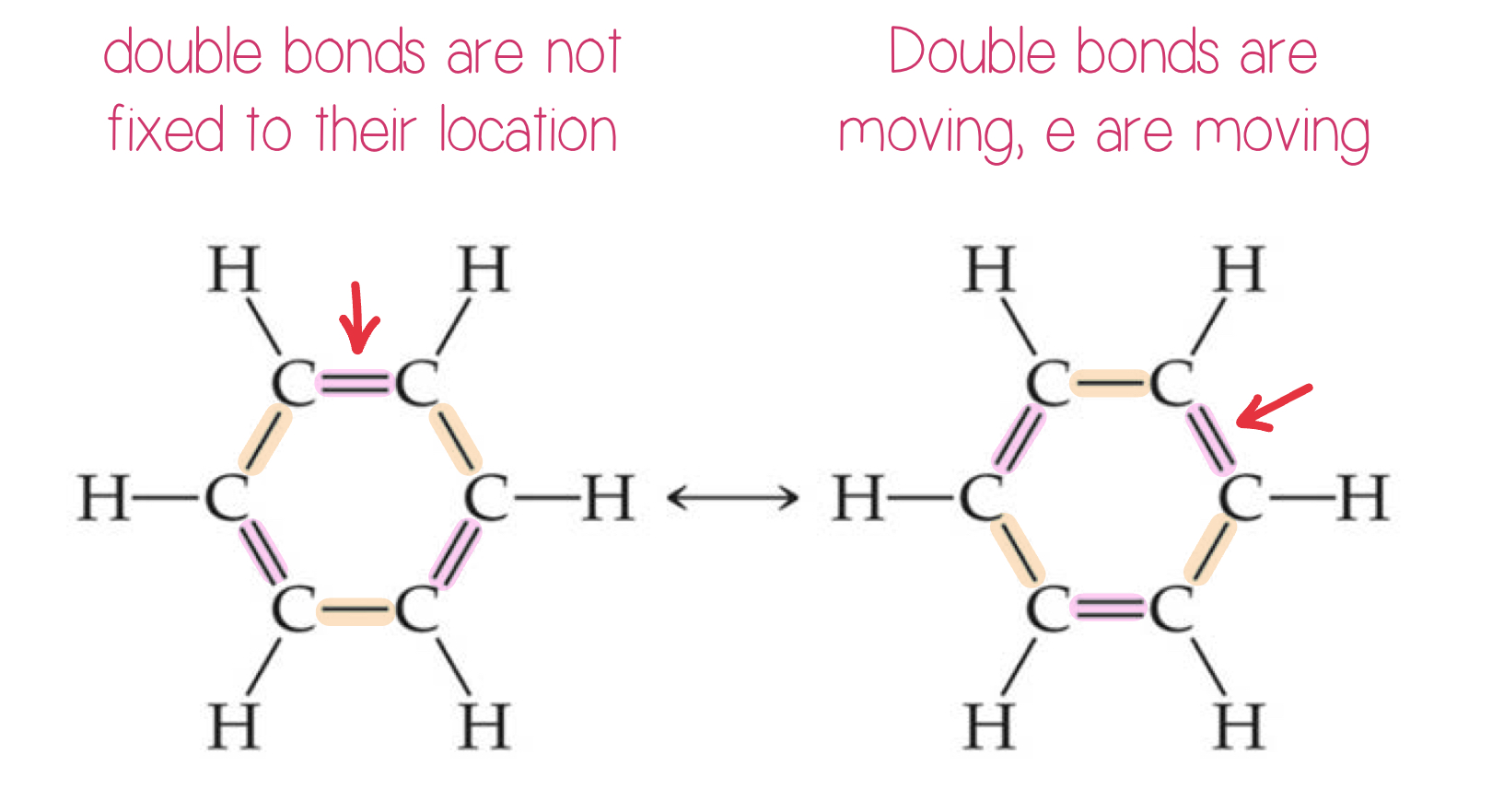
It is commonly depicted as a hexagon with a circle inside to signify the delocalized electrons in the ring.
Localized (fixed location) electrons are specifically on one atom or shared between two atoms.
Delocalized ( can move around) electrons are shared by multiple atoms.
Drawing the Result of Resonance Arrows
Arrow heads can only originate from the following:
Lone pairs
Pi bonds
NEVER have arrows originate from charges
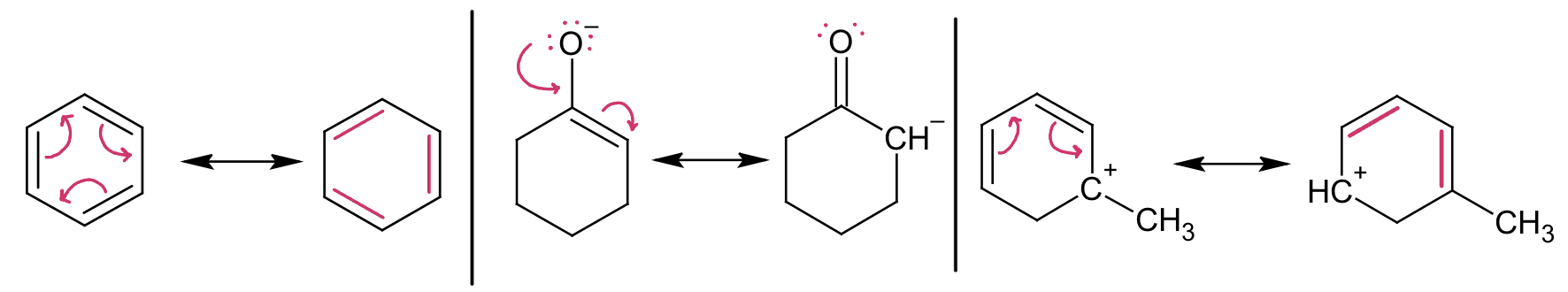
Resonance Rules
Never break a single bond.
Never exceed an octet.
Patterns We See in Drawing Resonance
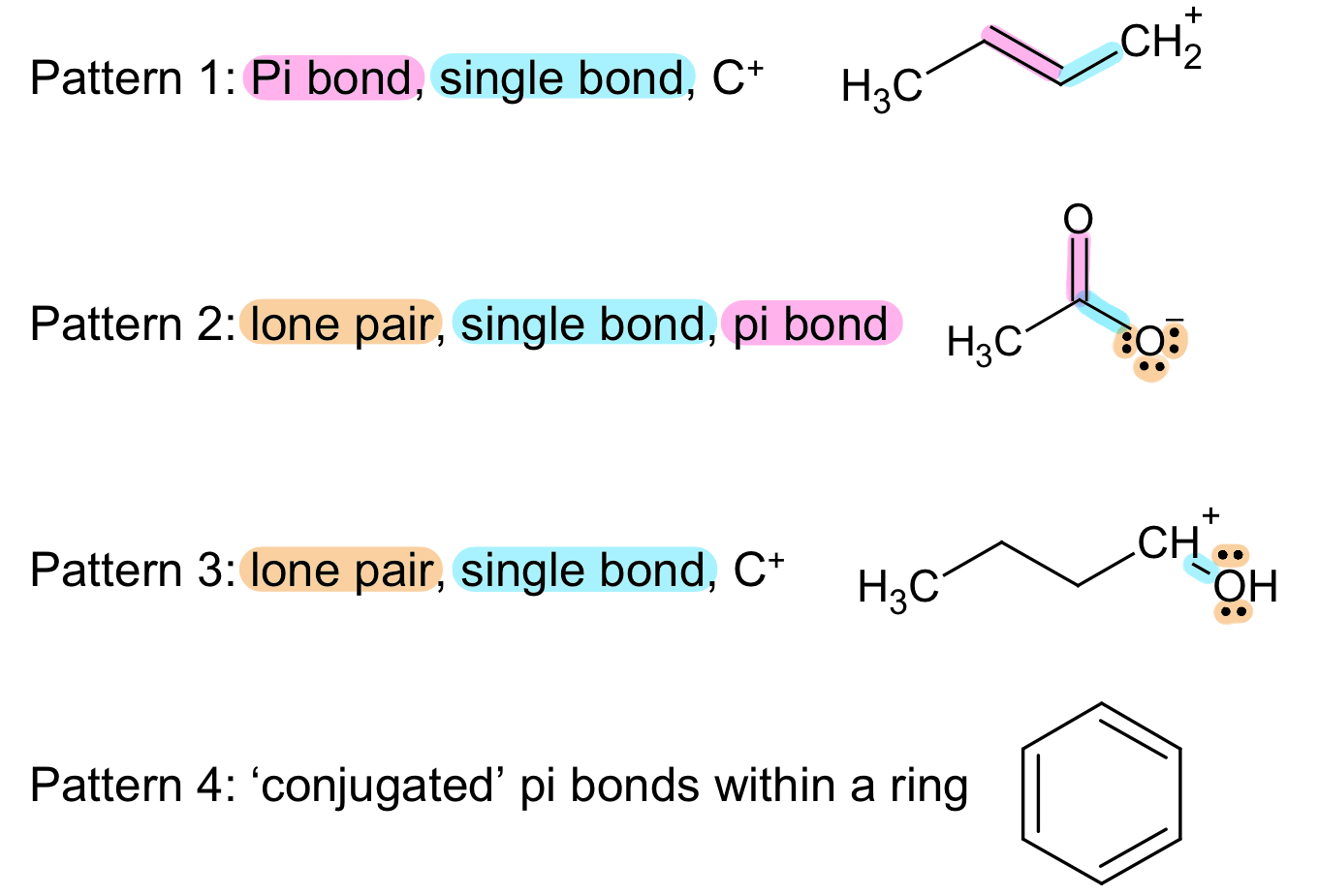
Most Representative Resonance Structure
Not all resonance structures are made equal.
Consider the following rules when ranking the importance of resonance structures:
The most important resonance structure has the greatest number of filled octets.
Resonance structures with the negative charge on the more electronegative atom is more significant.
Besides the above, all other resonance structures are equivalent.
Reference: Chemistry The Central Science (14th Edition)
 Knowt
Knowt
