Unit 4.2 Resonance, Shapes, and Giant Structures
4.2.1 Resonance Structures
Some atoms or elements have structures that don’t seem to fit with what you would expect their typical Lewis Structure to be
This can be explained by the delocalization of electrons
Delocalized electrons = electrons in a molecule, ion, or solid metal that are not permanently associated with one atom or covalent bond
Example:
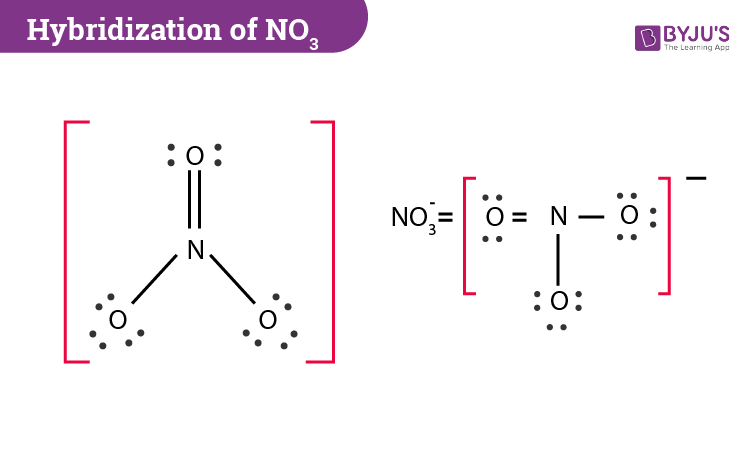
Nitrate (V) Ion
A molecule with 1 double bond and 2 single bonds
It has 3 possible Lewis Structures where the double bond moves around and is with each of the three oxygens

Since there are different possibilities, these Lewis Structures are also called Resonance Structures
So you would expect each of these Resonance Structures to have explicitly 1 double bond and 2 single bonds, right?
That’s not the case, however, because studies of the electron density and bond length show that all 3 bonds are equal in length
In fact, the electron density is spread evenly between the three oxygen atoms
The actual bond length for all of them is somewhere between a single and a double bond
The actual structure is something between all the resonance structures and is called a resonance hybrid
Resonance Structures of the Nitrate (V) Ion
Steps to Determine a Lewis Structure:
Count the # of Valence Electrons
Consider the Charge
Add more electrons for negative charges
Subtract electrons for positive charges
N + 3O + 1
5 + (3 × 6) +1
= 24 electrons
Draw a Skeleton
Put single bonds between atoms first
Generally, the least electronegative atom goes in the center
On the ends, put Hydrogens (because Hydrogen can only have 2 valence electrons and can only bond once) and Halogens
Subtract Skeletal Electrons from Valence Electrons
Use the remaining electrons to create Lone Pairs
OR additional bonds (double/triple) as needed
Remember the overall goal is to satisfy the Octet Rule
3 structures are possible for Nitrate (V) Ion, with 1 double and 2 single bonds
The negative charge is distributed throughout the ion and is depicted with the negative sign outside of the resonance brackets
Electron pairs rapidly oscillate between different positions, never really staying a single or a double bond for long
Criteria for forming resonance hybrid structures: molecules must have a double bond that is capable of migrating from one part of a molecule to another
In other words, when there are adjacent atoms with equal electronegativity and lone pairs of electrons that can move to another position in order for the double bonds to be in other positions
Ex. Carbonate Ion, Benzene, Ozone, and the Carboxylate Anion
4.2.2 Shapes of Molecules
VSEPR (Valence Shell Electron Pair Repulsion) Theory = A theory that predicts molecular shape and the angles between bonds based on the concepts:
All electron pairs and all lone pairs arrange themselves as far apart in space as possible
Lone Pairs repel more strongly than bonding pairs
Multiple bonds (double/triple) behave as single bonds
Domains = The regions of negative cloud charge
Steric Number (SN) = # of atoms + # of Lone Pairs around the central atom (same concept as Domains)
Can also be denoted as:
A = Central Atom
B = Bonded Pair
E = Lone Pair
ex. SN = 2 → AB2
ex. SN = 3 → AB3
ex. SN = 3 (but one of the domains is a lone pair) → AB2E1

Steric Number = 2
If SN = 2, then the angle between bonds is 180°
SN = 2 → AB2
Molecular Geometry = “Linear”
ex. BeCl2, CO2, HC≡CH

Steric Number = 3
If SN = 3, then the angle between the bonds is 120°
SN =3 → AB3
Molecular Shape = “Trigonal Planar”
ex. BF3 and CH2CH2 and CH2O


AB2E1
If one of the electron domains is a lone pair, then the bond angle is slightly less than 120° since lone pairs repulse more, pushing against the other two bonding pairs closer together
ex. SO2
Molecular Geometry = “Bent Linear”


Steric Number = 4
If SN = 4, then the angle between bonds is 109.5°
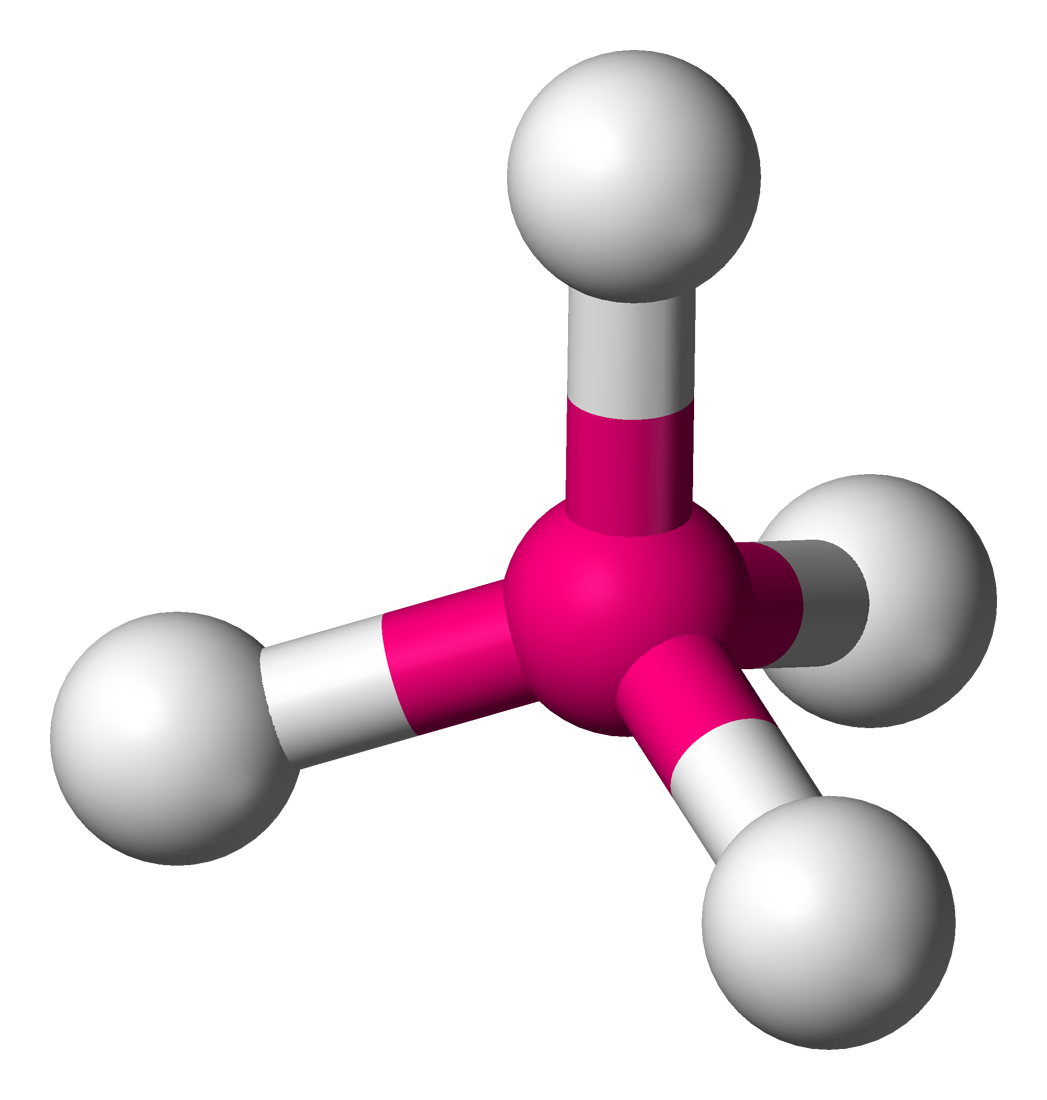
E.g. CH4, NH4+
SN = 4 → AB4
Molecular Geometry = “Tetrahedral”

AB3E1
If one of the electron domains is a lone pair, the bond angle is slightly less than 109.5° due to increased lone pair repulsion
ex. NH3
Molecular Geometry = “Trigonal Pyramidal”


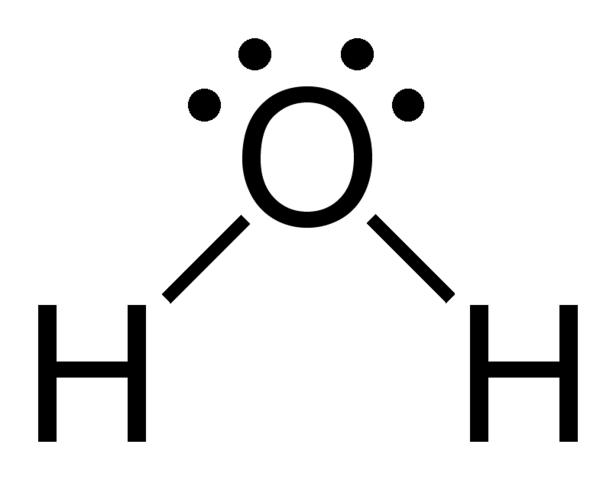
AB2E2
If 2 electron domains are lone pairs, bond angle also less than 109.5°
ex. H2O
Molecular Geometry = “Bent”

Summary


4.2.3 Predicting Shapes & Bond Angles
Draw Lewis Structure
Determine the number of bonding (B) and Lone Pairs (E) around the central atom (A)
Apply VSEPR Rules
Deduce shape and bond angle
4.2.4 Molecular Polarity
Bond Polarity ≠ Molecular Polarity
Previously, you learned that bond polarity was determined by the difference in electronegative felt between two bonded atoms
However, now you can determine if a molecule is polar or not
Consider:
The polarity of each bond in the molecule
How the bonds are arranged in the molecule
Note: Some molecules have polar bonds, yet are overall not molecularly polar since the polar bonds in the molecule are arranged in a way that the individual bond dipole moments cancel each other out
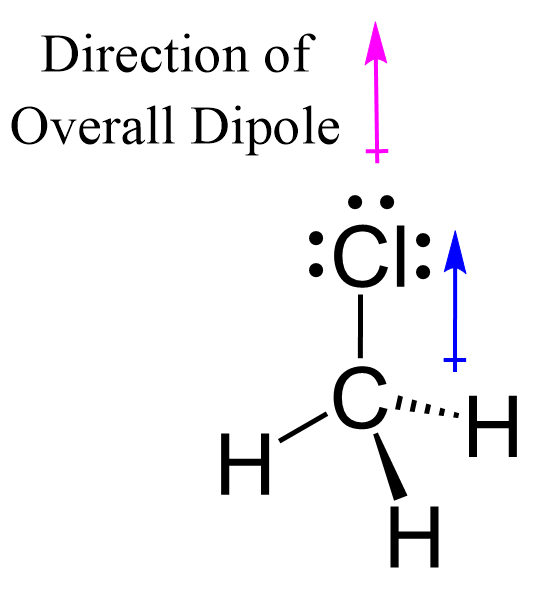
ex. CH3Cl vs. CCl4
CH3Cl
Has 4 polar covalent bonds that don’t cancel each other out
This means the molecule is polar overall
The overall dipole moment is pointing towards the electronegative chlorine atom
CCl4
Also has 4 polar covalent bonds, BUT the individual bond dipole moments cancel each other out
So CCl4 is a nonpolar molecule

4.2.5 Giant Covalent Structures
Giant Covalent Structures
Covalent Lattices
Covalent Bonds = Bonds between nonmetals in which electrons are shared between atoms
Giant Covalent Substances = Sometimes, a substance can't bond like a regular molecule. Instead, the bonds between atoms continue forever, forming a big lattice. There are no separate molecules in this situation, and all the nearby atoms are connected by covalent bonds.
ex. C
Allotrope = Different atomic or molecular arrangements of the same element in the same physical state
Graphite, diamond, buckminsterfullerene and graphene are allotropes of carbon
Giant Covalent Structures Examples
Diamond
Diamond is a giant lattice of carbon atoms
Each carbon is covalently bonded to 4 others in a tetrahedral geometry with a bond angle of 109.5°
This results in a giant lattice with strong bonds in all directions and causes diamond to be the hardest known substance
Graphite
Each carbon atom is bonded to 3 others in a layered structure of hexagons with a bond angle of 120°
The spare electron is delocalized and moves around in the space between the layers
All atoms in the same layer are held together by strong covalent bonds while the different layers are held together by weak intermolecular forces
![Graphite [SubsTech]](https://www.substech.com/dokuwiki/lib/exe/fetch.php?w=&h=&cache=cache&media=graphite_structure.png)
Buckminsterfullerene
Contains 60 carbon atoms
Each atom is bonded to 3 others by single covalent bonds
The fourth electron is delocalized so the electrons can migrate throughout the structure
This allows for the structure to be a semi-conductor
Has the same shape as a soccer ball, so it is nicknamed the football molecule

Graphene
Some substances infinitely covalent bond only in two dimensons, forming only layers
ex. Graphene
Graphene is a single layer of carbon atoms bonded in a repeating hexagonal pattern
It is so thin, 1 million times thinner than paper, that Graphene is actually considered 2D

Properties of Giant Covalent Structures
As always, different structures and bonding types have different effects on the physical properties of substances (ie. melting/boiling points, electrical conductivity, and solubility)
Covalent Bonding & Giant Covalent Lattice Structures
Giant Covalent Lattices:
Very High melting and boiling points
Large # of covalent bonds
A lot of energy is needed to break the lattice
Can be hard or soft
Hard (difficult to break their 3D network of strong covalent bonds)
Diamond
Silicon (IV) Oxide
Soft (forces between carbon layers are weak)
Graphite
(Graphene is strong, flexible, and transparent, making it a very useful material)
Insoluble in water (Most)
Do NOT conduct electricity (Most)
The some that do have delocalized electrons:
Graphite
Graphene
Buskminsterfullerene (semi-conductor)