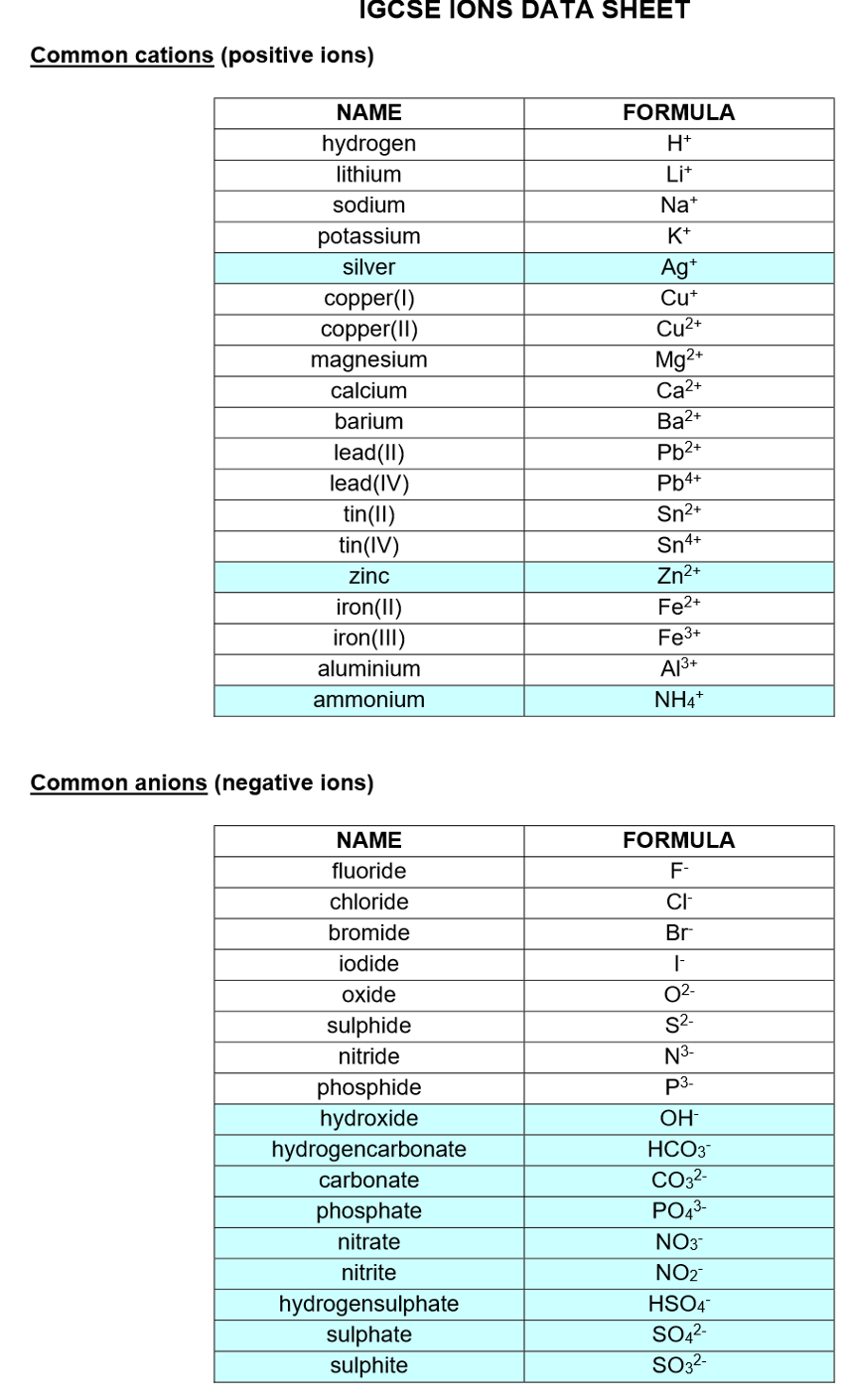
Periodic table notes
Atomic Structure (Mainly Review)
Key Terms
Atom: The basic unit of matter, consisting of a nucleus (made up of protons and neutrons) surrounded by electrons in shells. Atoms are the building blocks of all substances.
Molecule: A group of two or more atoms bonded together, representing the smallest unit of a chemical compound that can participate in a chemical reaction.
Isotopes: Species with same number of protons different number of neutrons
Structure of an Atom
Atoms are composed of three main subatomic particles: protons, neutrons, and electrons. Each of these has specific properties:
Protons (p): Positively charged (+1) and have a relative mass of 1. Protons are found in the nucleus of the atom and determine the atomic number (which defines the element).
Neutrons (n): Neutrally charged (0) and have a relative mass of 1. Neutrons are also located in the nucleus and contribute to the mass number of an atom.
Electrons (e): Negatively charged (-1) and have a negligible mass (approximately 1/2000th of the mass of a proton). Electrons are found orbiting the nucleus in energy levels (shells).
The nucleus of the atom is composed of protons and neutrons, and is dense and positively charged. Electrons, being much lighter, orbit around the nucleus in specific energy levels or shells.
Atomic Terms
Atomic number: The number of protons in the nucleus of an atom, which determines the identity of the element.
Mass number: The total number of protons and neutrons in an atom’s nucleus.
Isotopes: Atoms of the same element that have the same number of protons (same atomic number) but different numbers of neutrons (different mass numbers).
Relative atomic mass (A_r): The weighted average mass of an atom of an element, taking into account the abundances of all its isotopes, relative to 1/12th the mass of a carbon-12 atom.
Calculating Relative Atomic Mass
The relative atomic mass (A_r) of an element can be calculated using the abundances and masses of its isotopes. The formula for this calculation is:

For example, chlorine has two main isotopes, Cl-35 and Cl-37. If their relative abundances are known, you can calculate chlorine’s relative atomic mass as a weighted average.
1d Introduction to the Periodic Table
Periodic Table Arrangement
Atomic number: Elements in the Periodic Table are arranged in order of increasing atomic number (the number of protons in an atom’s nucleus).
Groups: Vertical columns in the Periodic Table. Elements in the same group have the same number of electrons in their outer shell, which gives them similar chemical properties.
Periods: Horizontal rows in the Periodic Table. As you move across a period, the number of protons increases, and electrons are added to the same energy level (shell).
Electron Configurations
The electron configuration of an atom describes the arrangement of electrons in shells around the nucleus.
The elements in the first 20 positions of the Periodic Table follow a simple rule: electrons fill up the lowest energy levels first (closest to the nucleus). This is often written as a series of numbers that represent the electrons in each shell:
Hydrogen (1): 1
Helium (2): 2
Lithium (3): 2, 1
Calcium (20): 2, 8, 8, 2 (after the first shell is filled, electrons fill the second shell, then the third, and so on).
The position of an element in the Periodic Table is related to its electron configuration. Elements in the same group have the same number of outer-shell electrons, which influences their reactivity.
The charges of ions can also be deduced from the element’s position. For example, Group 1 elements lose one electron to form +1 ions, while Group 7 elements gain one electron to form -1 ions.
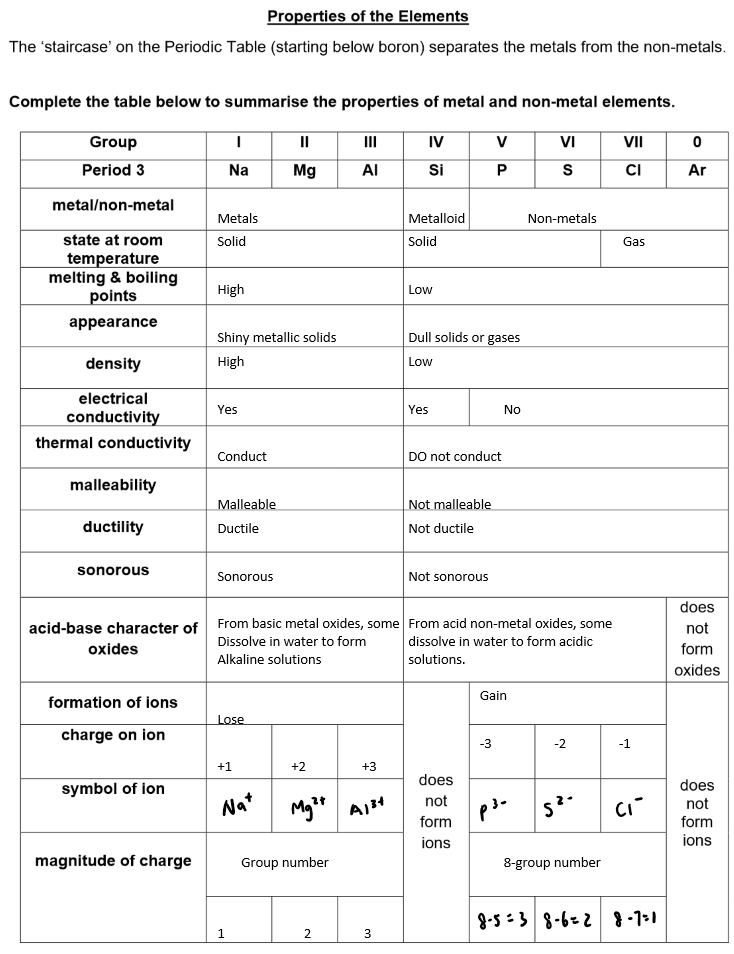
Classification of Elements
Elements can be classified as metals or non-metals based on their properties, such as:
Electrical conductivity: Metals generally conduct electricity well, while non-metals do not.
Acid-base character of oxides: Metals tend to form basic oxides, while non-metals form acidic oxides. Amphoteric oxides (such as aluminum oxide) can react with both acids and bases.
The Periodic Table is divided into metals on the left and non-metals on the right, with a diagonal dividing line separating them.
Metal vs Non-metal Identification
Elements can be identified as metals or non-metals based on their position in the Periodic Table:
Metals are typically found on the left-hand side and in the center of the table (transition metals).
Non-metals are found on the right-hand side of the table (e.g., carbon, nitrogen, oxygen).
The properties of elements (such as malleability, conductivity, and luster) help distinguish metals from non-metals.
Electron Configuration and Position
The electronic configuration of a main group element (elements in Groups 1, 2, and 13-18) determines its position in the Periodic Table.
The number of outer-shell electrons determines the group number. For example, sodium (Na) has an electron configuration of 2, 8, 1, which places it in Group 1.
The number of electron shells used corresponds to the period number. For example, sodium (Na) has 3 electron shells, so it is in Period 3.
Group Similarities
Elements in the same group of the Periodic Table have similar properties because they have the same number of electrons in their outermost shell (valence electrons).
For example, the alkali metals (Group 1) are all highly reactive metals that lose one electron to form +1 ions, while the halogens (Group 7) gain one electron to form -1 ions.
Noble Gases (Group 0)
The noble gases (Group 0 or 18) are unreactive because they have complete outer electron shells, making them stable and inert.
Because their outermost shell is full, noble gases do not need to gain, lose, or share electrons, which is why they do not readily participate in chemical reactions.
Examples of noble gases include helium (He), neon (Ne), and argon (Ar).