1.1: Chemical elements and biological compounds
Inorganic ions
Inorganic ions are ions without carbon, and living organisms require small amount of these. They can also be called electrolytes or minerals.
There are two types, micronutrients - needed in minute concentrations and macronutrients - needed in small concentrations.
There are four main ones:
Magnesium - a constituent of chlorophyll and essential in photosynthesis. Without this, chlorophyll cannot be produced so leaves appear yellow, a condition known as chlorosis. It is also needed for bones.
Iron - a constituent of haemoglobin, which is necessary for oxygen transport around the body using red blood cells. Lack of iron leads to anaemia.
Phosphate ions - used for the synthesis of nucleotides, such as ATP, and phospholipids, which form cell membranes.
Calcium - required for bones, teeth and plant cell walls.
There are five lesser known:
Chlorine - necessary to maintain electrical neutrality across membranes
Nitrate - component of DNA, RNA, ATP, phospholipids, and nucleotides
Potassium - A factor in protein synthesis and respiration
Sodium - Needed to maintain active transport across cell membranes, and electrical balance
Sulphate - Forms bridges between polypeptide chains
Water
Water is necessary for cells, and is 65% - 95% the mass of plants and animals - 70% in humans. It is a medium for metabolic reactions.
Structure
Water is a dipole, meaning it has a positively charged end (hydrogen) and negatively charged end (oxygen). These are represented with a delta symbol, as they are only partial charges.
Hydrogen bonds can form between water molecules, attracted to the opposite charges on each side. Hydrogen bonds are weak in small amounts, but in large groups makes water difficult to separate and gives water most of its properties.
Properties
Solvent - water is known as the universal solvent due to its effectiveness in this role. This is due to its dipole molecules, which attracted charged particles and polar molecules, such as ions and glucose. They dissolve, and the chemical reactions take place in solution.
Transport - in plants, xylem vessels transport minerals in the xylem. However, it only dissolves polar molecules, therefore the phloem has to transport molecules such as lipids.
Metabolite - water is used in many chemical reactions in the body, usually during hydrolysis, where it splits molecules. It is also needed for photosynthesis. Additionally, it is a common product, in reactions such as condensation.
High specific heat capacity - this translates to a large amount of energy is needed to raise its temperature. This is due to hydrogen bonds restricting movement, therefor kinetic energy is resisted. However, in small amounts water will raise temperature fast. This is necessary for large bodies of water, as it keeps aquatic environments stable. It also prevents enzymes from denaturing within the body.
High latent heat of vaporisation - following from the last property, high amounts of heat energy are required to change water to a vapour. This helps with temperature control, where sweat evaporates from the skin to help cool the body.
Cohesion - this occurs when many hydrogen bonds stick together in a lattice, strengthening the bonds. This is necessary in plants, as it allows columns of water to create xylem vessels.
High surface tension - this is caused by cohesion between surface water molecules, and water has the highest surface tension at average temperatures, other than mercury. This allows insects to walk on water, such as pond skaters.
High density - water is denser than air, providing support and buoyancy for aquatic ecosystems. Additionally, ice is less dense than water due to the distance between the hydrogen bonds, allowing it to float. Ice insulates water, preventing further freezing and allowing organisms beneath it to survive.
Transparency - this allows light to pass through, so underwater plants are able to photosynthesise.
Carbohydrates
This are organic compounds containing carbon, oxygen and hydrogen. The smallest form is a monosaccharide, which combines with another to form a disaccharide, and combine with more to form polysaccharides.
Monosaccharides
They have the general formula (CH2O)n, with n representing carbon. The amount of carbon molecules also determine the name of monosaccharides.
Monosaccharides are drawn in rings, with carbon at almost every point.
The carbons in a monosaccharide make a ring when dissolved in water, which can alter their binding to make straight chains, rings and chains in equilibrium.
Glucose is an example of a monosaccharide, with two isomers: alpha glucose and beta glucose. Beta glucose has the hydrogen on ring 1 below, while alpha has it above.
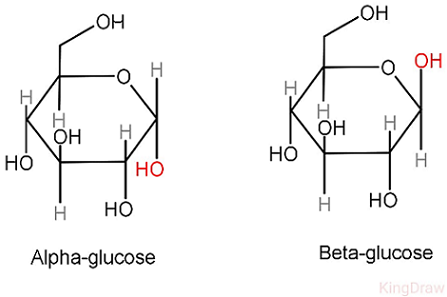
Functions of monosaccharides include:
They are ‘building blocks‘ for larger molecules, such as polysaccharides.
The bonds they create are a source of energy in respiration, as when carbon-hydrogen and carbon-carbon bonds are broken they release high amounts of energy, which creates large amounts of ATP.
They are intermediates in reactions, such as photosynthesis and respiration.
Constituents of nucleotides, such as ribose in RNA.
Disaccharides
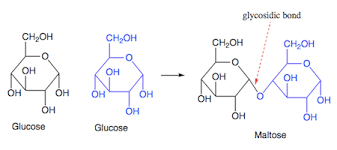
These are two monosaccharides bonded with a glycosidic bond, formed after a condensation reaction. This reaction removes water.
The bond is between C1 and C4, making it a 1,4 glycosidic bond
Examples of disaccharides are:
Sucrose, which is used in table sugar and found naturally in foods.
Lactose, in mammalian milk.
Polysaccharides
These are complex polymers, with chains linked by glycosidic bonds. Glucose is necessary for energy in cells, and must be converted to a different process to avoid decreasing water potential. It is also to make the glucose more compact.
Starch
This is used for storage in plants, and found in high concentrations in seeds.
Starch is made from two alpha glucose molecules bonded in different ways, the polymers known as amylose and amylopectin:
Amylose is linear and unbranched, bonded with 1,4 glycosidic bonds. This forms a chain, which coils into a helix
Amylopectin has 1,4 glycosidic bonds, but also 1,6 bonds which form branches. These occur every 24-30 molecules and fit inside the amylose.
Glycogen
The main storage product in animals.
It has 1,4 and 1,6 alpha glucose glycosidic bonds, the latter occurring every 8-10
Cellulose
Found in plant cell walls - a structural polysaccharide.
Made from long chains of beta-glucose molecules, which form 1,4 glycosidic bonds. Each molecule is flipped 180 degrees in relation to the other.
Hydrogen bonds form between adjacent parallel chains, improving the structural integrity. These form bundles known as microfibrils, which are held in bundles called fibres.
Cells have several layers of fibres, and are also freely permeable due to spaces between the fibres. Water and solutes are able to penetrate these spaces in the cell wall.
Chitin
This is a structural polysaccharide found in insect exoskeletons and fungal cell walls.
It has 1,4 linked monomers which are rotated 180 in degrees in relation to one another.
Parallel chains are cross-linked using hydrogen bonds, forming microfibrils.
It has amino acid derived groups added, making it a heteropolysaccharide.
It is waterproof, strong and lightweight.
Lipids
Lipids contain carbon, hydrogen and oxygen, but have less oxygen in proportion to the other elements.
They are non-polar, therefore insoluble in water. However they dissolve in organic solvents, such as alcohols.
Properties of lipids
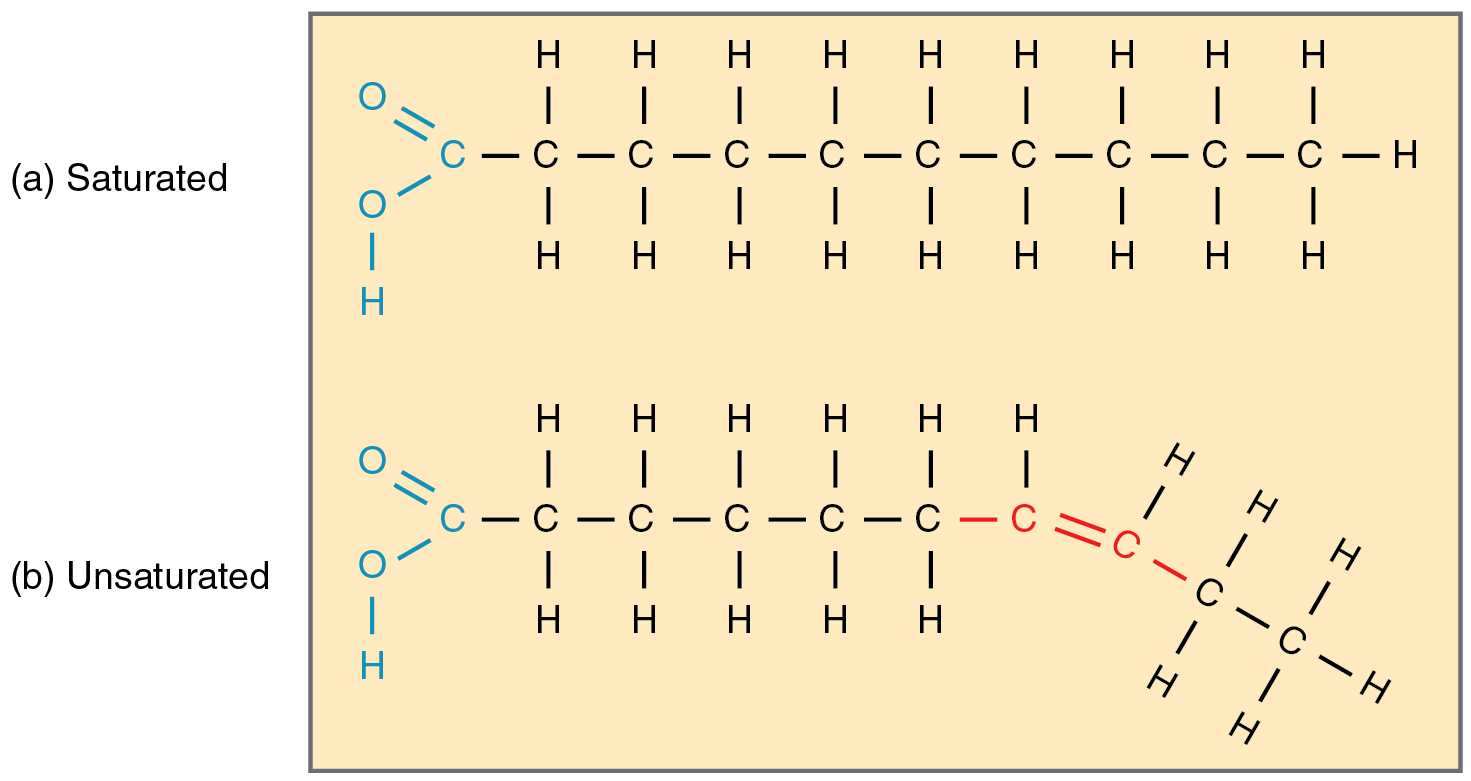
Saturated
Saturated lipids have only single bonds, meaning each carbon is joined to the maximum amount of hydrogens.
These molecules align readily, causing them to be semi-solid at body temperature and therefore useful in mammalian storage. Animal lipids often contain fatty acids.
In the small intestine, these form LDLs (low density lipoproteins) which can build up. Atheroma (fatty material) is deposited in the coronary arteries, restricting blood flow and oxygen delivered to the heart.
This can cause angina, heart attacks, myocardial infarctions, atherosclerosis and hypertension.
Unsaturated
If there are any double bonds between the carbons, these makes the molecule unsaturated and causes a kink in the chain.
These molecules cannot align uniformly and therefore cannot solidify at room temperature. Plant lipids are often oils, such as olive and sunflower oil.
If there is one double bond, they are monounsaturated. If there are many, they are polyunsaturated.
Unsaturated fats form HDLs (high density lipoproteins) which carry away harmful fats to the liver for disposal. The higher the HDL to LDL ratio, the lower their risk of cardiovascular and coronary heart disease.
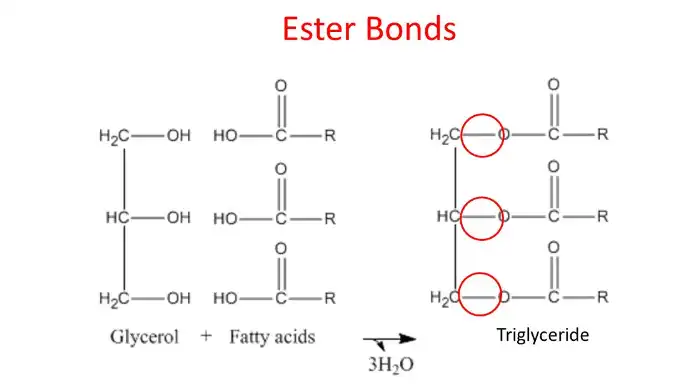
Triglycerides
These are formed by one glycerol molecule and three fatty acid molecules, with the fatty acid components varying.
They join using a condensation reaction, losing three water molecules and forming ester bonds between the glycerol and each fatty acid.
They can be used as energy reserves in plants and animals, thermal insulation, protection against physical damage and production of metabolic water when oxidised.
Phospholipids
Phospholipids have one phosphate and glycerol end, joined to two fatty acids.
The glycerol and phosphate end has lots of oxygen atoms, making it hydrophilic and polar, while the fatty acid tails have no oxygen and are therefore hydrophobic and non-polar.
They can be used in biological membranes and electrical insulation of nerve cell axons.
Waxes
They can only melt above 45 degrees.
They are used in waterproofing, for example the insect exoskeleton and the leaf’s cuticle to reduce water loss.
Proteins
Proteins contain carbon, hydrogen, oxygen and nitrogen. They can also contain sulphur and phosphorus.
Proteins are polymers made up of amino acids, their chains known as polypeptides. There are around 20 different amino acids used to make up proteins, their sequences determining the structure and therefore creating thousands of different proteins.
Amino acids have the same basic structure, surrounding a carbon atom:
On the left of the molecule is the amino group, -NH2, which is known as the N terminal.
On the other end is the carboxyl group, -COOH, known as the C-terminal.
A hydrogen atom.
The R group at the top of the molecule, which differs in each amino acid and determines the structure.
At cell pH, 7, the amino group gains hydrogen and becomes positive while the carboxyl group loses hydrogen and becomes negative. This means it has two different charges, making it a zwitterion.
Amino acids bond by the carboxyl and amino groups joining, eliminating a water molecule. This is a condensation reaction, and forms a peptide bond. This molecule would be known as a dipeptide.
Depending of the order of the bond, the dipeptide will have different properties.
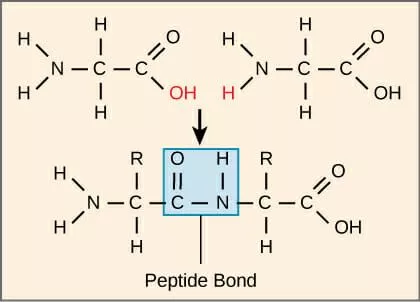
Protein structure
Protein structure is described using different levels of organisation.
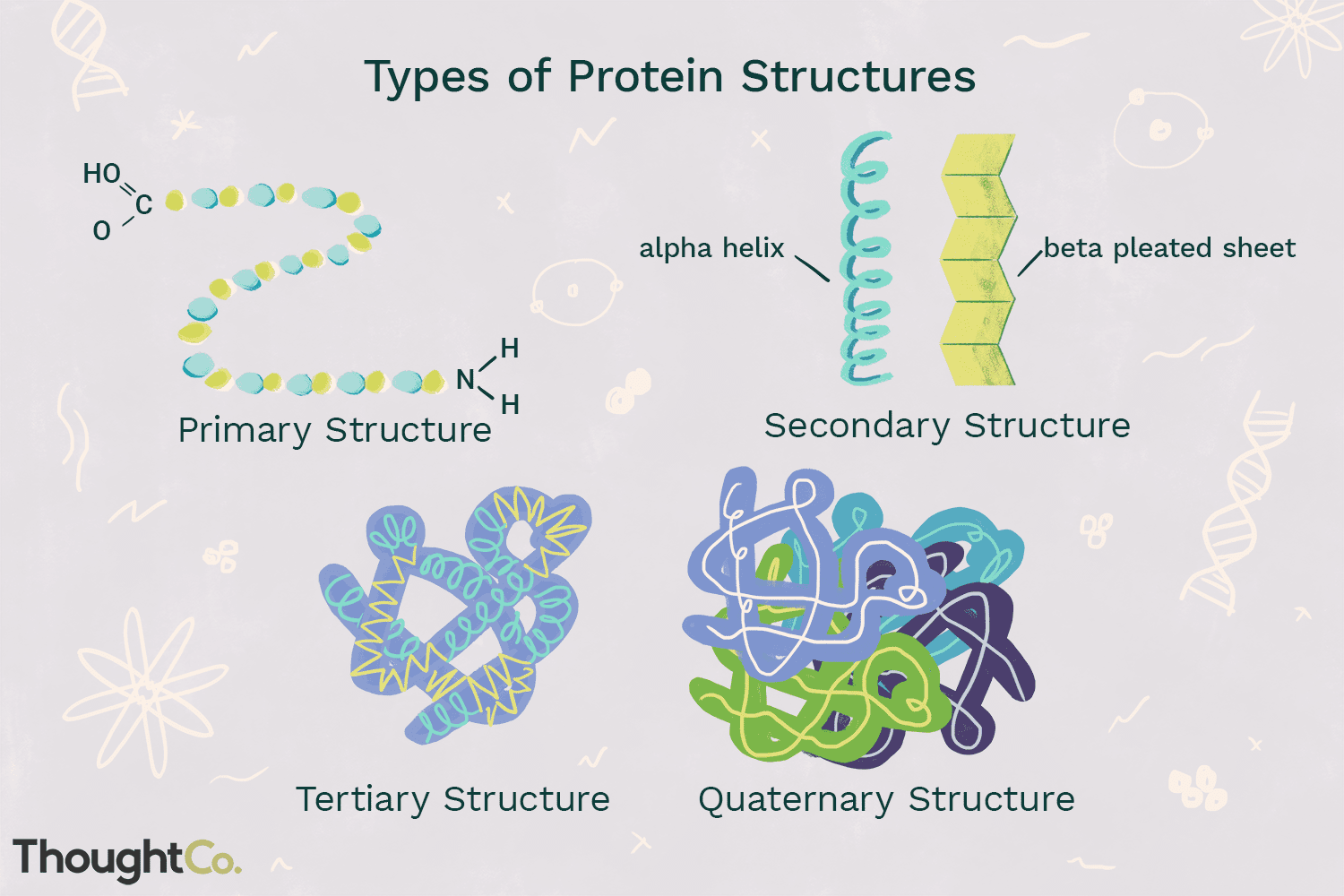
Primary structure
This is the order of the amino acids, which determines the structure of the protein. This is determined by the base sequence on one strand of the DNA molecule
Secondary structure
This shape is formed by hydrogen bonding between peptide bonds along a polypeptide chain. This twists the chain into a 3D structure, of which there is two types:
The alpha-helix, which twists to form a helix. An example is the protein keratin.
The beta-pleated sheet, which resembles folded cardboard. This is less common. An example is the protein fibroin in silk.
Tertiary structure
The alpha helix can twist further to give a more complex and compact 3D shape. There are many bonds that maintain this structure:
Hydrogen bonds.
Ionic bonds (salt bridges).
Disulphide bonds (only formed if the protein contains sulphur).
Hydrophobic interactions.
Examples are enzymes, which lower activation energy and myoglobin, which are found in striated muscles and supplies oxygen to cells within muscles.
Quaternary structure
These are polypeptide chains working together, and some are non-functional unless they combine.
Examples are:
Haemoglobin, which transfers blood around the body and bonds with non-protein groups.
Insulin, which helps cells absorb sugar, combines with other protein groups.
Globular and fibrous proteins
Fibrous proteins have long, thin molecules that makes them insoluble in water. They are mainly used for structure, such as in bone, as they are strong and tough. The polypeptides of fibrous proteins are in parallel chains which bond together with cross-links, forming long fibres.
An example is collagen, which is used in tendons. They form tropocollagens, which are three identical chains twisted around each other and held in place by hydrogen bonds.
Another is keratin, which is in hair and nails due to its strength.
Globular proteins are compact and form spherical molecules, making them soluble in water.
An example is haemoglobin, a complex molecule which carries oxygen around the body. It consists of four polypeptide chains with iron at the centre.
Another is enzymes, which are used to lower the activation energy of reactions in the body.
Tests
Test for reducing sugars
The Benedict’s test detects reducing sugars, as Benedict’s solution reduces the copper (2) ions to copper (1) ions, causing the solution to change from blue to brick red if positive.
The solution must be equal to the amount of Benedict’s solution when added, and heated to at least 70 degrees.
This is a qualitative test, therefore not showing the actual concentration of the reducing sugar.
Test for starch
The iodine-potassium iodine test detects starch, as the iodine interacts with it, changing from orange-brown to blue-black if positive.
This is a qualitative test, although the depth of the blue-black colour can indicate the concentration.
Test for fats and oils
The emulsion test mixes ethanol with the solution, which will dissolve any lipids present, and then shaken with an equal volume of water, which causes the water insoluble lipids to leave the solution, forming a cloudy white emulsion.
This is a qualitative test.
Test for proteins
The biuret test detects proteins, as when biuret reagent is added as blue copper hydroxide interacts with the peptide bonds in the protein, changing the colour to purple.
This is a qualitative test, although the darkness of the purple indicates a high concentration of proteins.