Guide: AP Environmental Science (U1- some of U4)
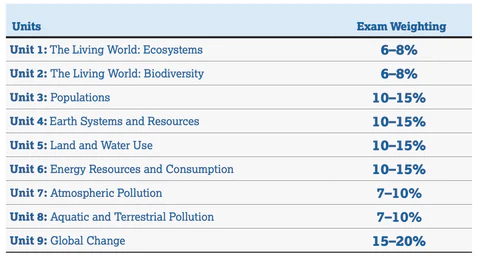
Resources to review :
https://www.youtube.com/@Mr.Smedes
https://knowt.com/note/1607cb1a-986f-435d-9f60-91d9b9b77d38/Ultimate-Guide-AP-Environmental-Science
Chapter 1: The Living World (Ecosystem)
1.1: Introduction to Ecosystems
Ecosystem: A community of living (biotic) organisms interacting with the non-living (abiotic) components of their environment as a system through various nutrients and energy cycles.
Biological Populations and Communities
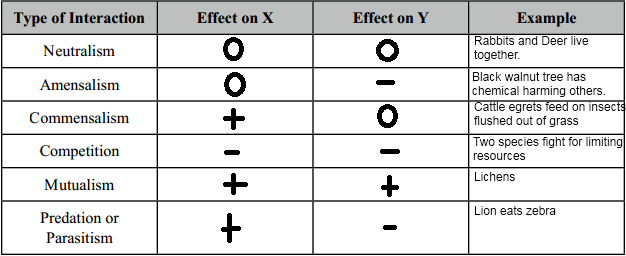
O: neutral impact
+: positive impact
- : negative impact
Commensalism: The interaction between two species whereby one organism benefits and the other species is not affected.
Competition:
It is the driving force of evolution whether it is for food, mating partners, or territory.
Intraspecific: Competition between members of the same species.
Interspecific: competition between members of different species.
Competition is prominent in predator–prey relationships, with the predator seeking food and the prey seeking survival.
Limiting Factors
Limiting Factor: Any abiotic factor that limits or prevents the growth of a population
Limiting factors in terrestrial ecosystems may include:
the level of soil nutrients
the available amount of water and light
the temperature
In aquatic ecosystems, major limiting factors may include:
the pH of the water,
the amount of dissolved oxygen, light, or
the degree of salinity.
1.2: Terrestiral Biomes:
Determinants: temperature and precipitation
Biomes: regional or global biotic communities characterized by dominant forms of plant life and the prevailing climates
Deserts: Defined in terms of the amount of rainfall they receive, not temperature.
Forests
Tropical Rainforests
Temperate Deciduous Forests: four distinct seasons, with trees shedding their leaves in the fall and regrowing them in the spring, resulting in high biodiversity and rich nutrient cycling.
Temperate Coniferous Forest
Taiga or boreal forest
Grasslands: lands dominated by grasses rather than by large shrubs or trees.
Tropical grasslands or savannas
Temperate Grasslands
Tundra:
extremely low temperatures and conditions
little precipitation
low biotic diversity: specilized animals only
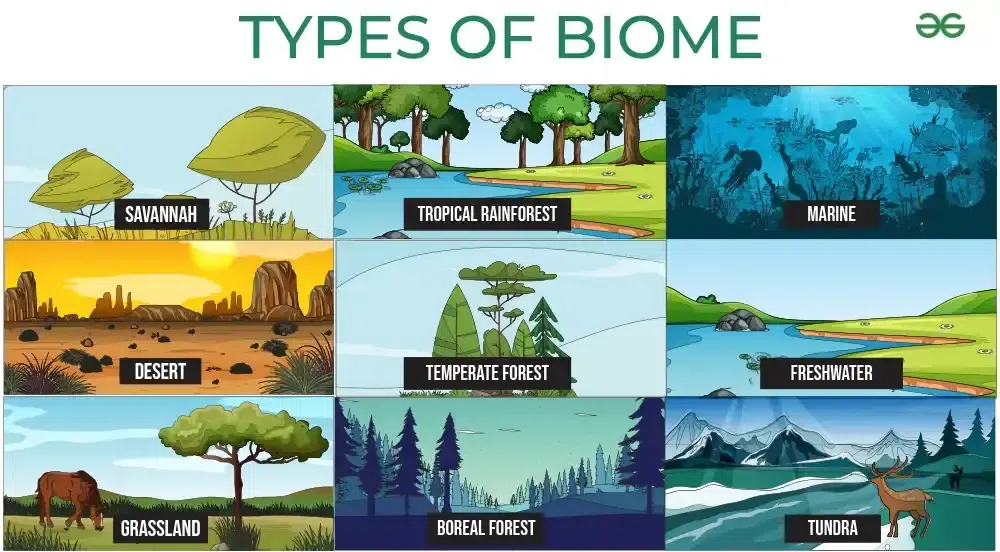

1.3: Aquatic Biomes


Ocean Zones
Littoral Zone: intertidal zone, it is the part of the ocean that is closest to the shore.
Neretic Zone: sublittoral zone, this zone extends to the edge of the continental shelf.
Photic Zone: The uppermost layer of water in a lake or ocean that is exposed to sunlight down to the depth where 1% of surface sunlight is available.
Antarctic, marine, lakes, wetlands, rivers, coral reefs and streams comprise aquatic biomes.
Aquatic organisms get nutrients from water.
Water's thermal capacity is high, most aquatic organisms don't need to regulate temperature.
Water screens out UV radiation.
Convection: The circular motion that occurs when warmer air or liquid rises, while the cooler air or liquid sinks.
Air and oceanic currents carry heat from the equator to the poles.
Wind patterns caused by tropical air flowing to the polar regions drive surface ocean currents.
Temperature and density control deep-water, density-driven currents.
Deeper ocean waters are colder and denser than near-surface waters.
Cold, salty water sinks, while warmer water rises.
This water sinks because it loses heat and becomes cooler and denser.
Riparian areas: These are lands adjacent to creeks, lakes, rivers, and streams that support vegetation dependent upon free water in the soil.
Vegetation consists of hydrophilic (water-loving) plants and trees.
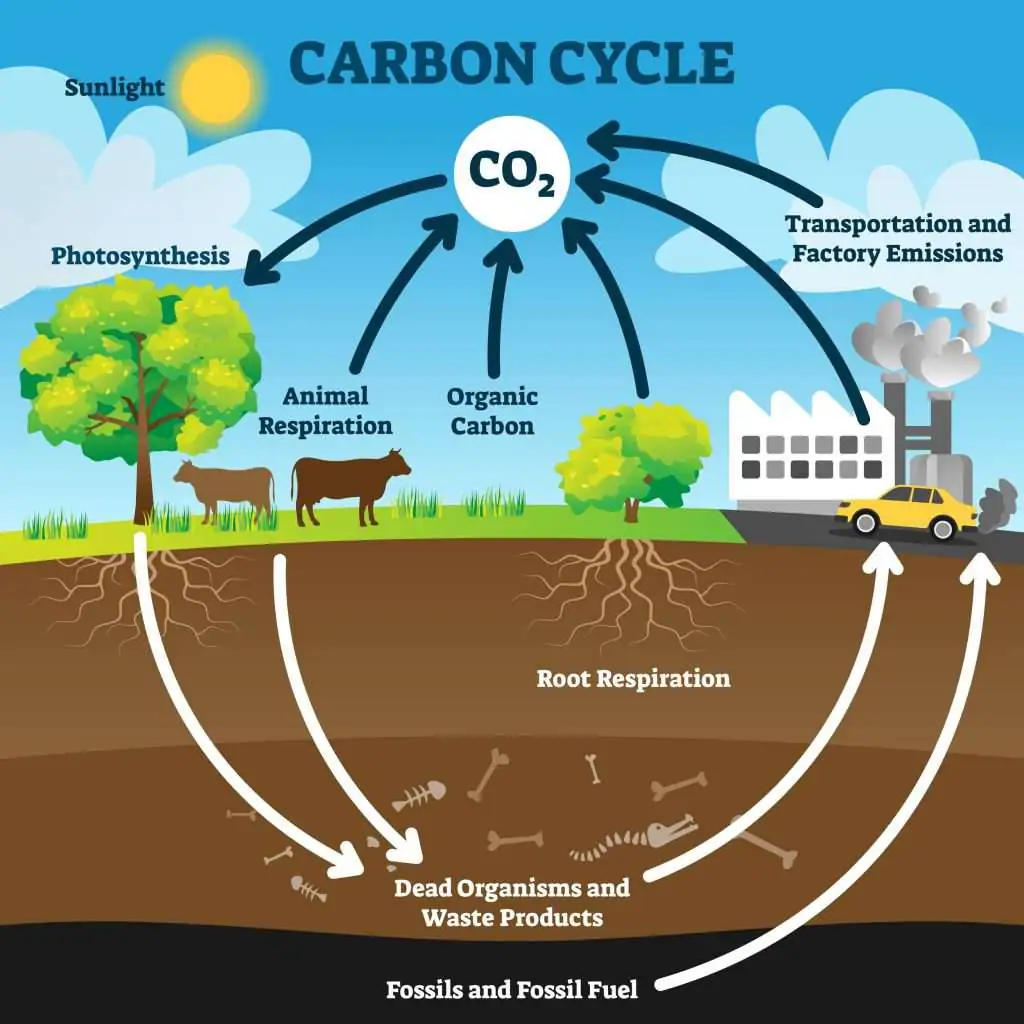
1.4: Carbon Cycle
Carbon: basic building block of life (CHNOPS); fundamental element found in carbohydrates, fats, proteins, and nucleic acids.
carbon dioxide, makes up less than 1% of the atmosphere.
Due to rising CO2 concentrations, oceanic acidity may slow the natural precipitation of calcium carbonate, reducing the ocean's capacity to absorb CO2.
Positive feedback loop
The major reservoirs or “sinks” of carbon:
Plant Matter
Terrestrial Biosphere: Forests store about 90% of the planet’s above-ground carbon and about 75% of the planet’s soil carbon.
Oceans: CO2 dissolved in seawater is utilized by phytoplankton and kelp for photosynthesis.
Human Impact on the Carbon Cycle
Industrial Revolution
deforestation
combustion of fossil fuels released carbon stored in long-term carbon sinks, causing climate change and the following environmental impacts:
increased acidity of oceans
increase in atmospheric particulate matter
increased rate of melting of long-term water storage
stronger and more frequent storm events
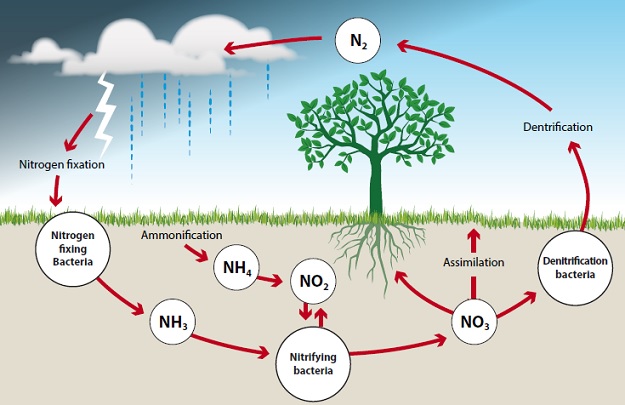
1.5: Nitrogen Cycle
Nitrogen makes up 78% of the atmosphere.
One of the CHNOPS elements: essential element needed to make amino acids, proteins, and nucleic acids.
Though atmospheric nitrogen (N2) is abundant
Nitrogen increases water acidification, eutrophication, and toxicity.
It is needed for photosynthesis and plant growth in chlorophyll.
Nitrogen Fixation: Atmospheric nitrogen is converted into ammonia (NH3) or nitrate ions (NO3–), which are biologically usable forms of nitrogen.
nitrogen-fixing bacteria known as Rhizobium.
Nitrification: Ammonia (NH3) is converted to nitrite (NO2–) and nitrate (NO3–), which are the most useful forms of nitrogen to plants.
Assimilation: Plants absorb ammonia (NH3), ammonium ions (NH4+), and nitrate ions (NO3–) through their roots.
Ammonification: Decomposing bacteria convert dead organisms and wastes, which include nitrates, uric acid, proteins, and nucleic acids, to ammonia (NH3) and ammonium ions (NH4+)—biologically useful forms.
Denitrification: Anaerobic bacteria convert ammonia into nitrites (NO2–), nitrates (NO3–), nitrogen gas (N2), and nitrous oxide (N2O) to continue the cycle.
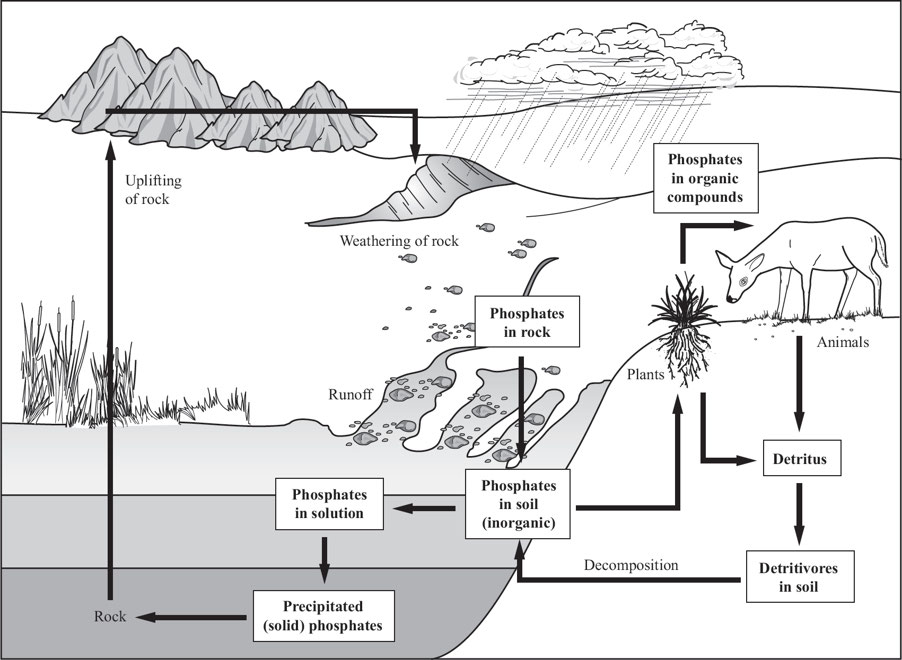
1.6: The Phosphorous Cycle
Part of CHNOPS
Phosphorus is not found in the atmosphere;
primary sink for phosphorus is in sedimentary rocks; form of solid
slowly released: by weathering (acid, rain, water and wind) or erosion
often a limiting factor for soils due to its low concentration and solubility
key element in fertilizer
Humans have impacted the phosphorus cycle in several ways, as follows:
Allowing runoff from feedlots, fertilizers, and municipal sewage plants to collect in bodies of water (lakes, streams, oceans)
leading to Eutrophication
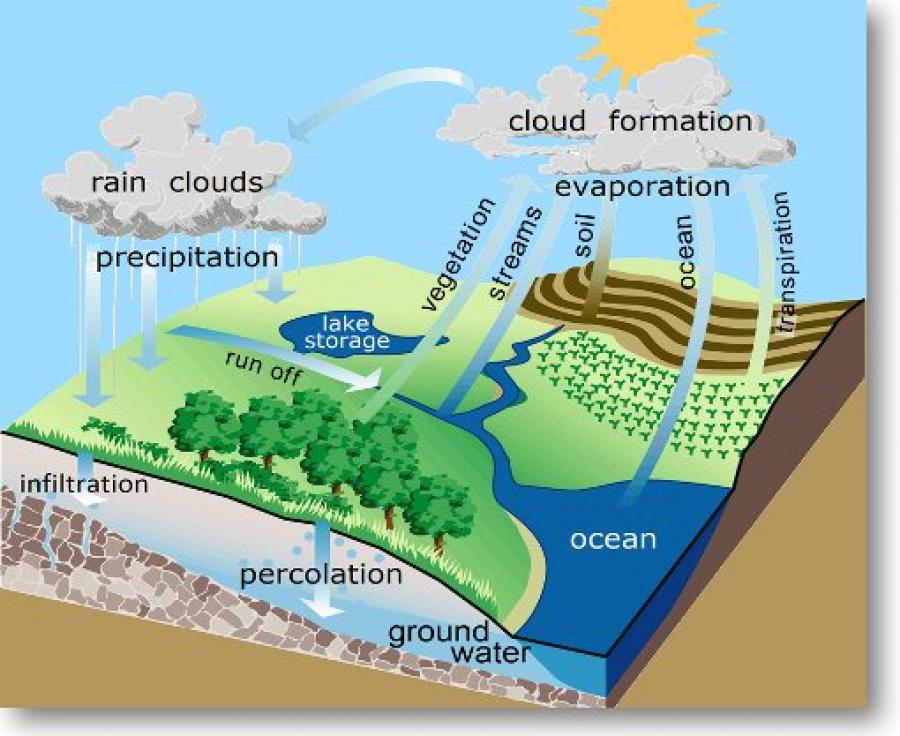
1.7: The Hydrologic Cycle
Water cycle: It is powered by energy from the sun, which evaporates water from bodies of water and plants
Warm air holds more water vapor than cold air.
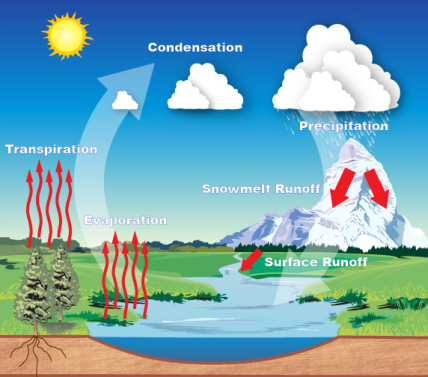
Condensation: The conversion of a vapor or gas to a liquid
Evaporation: The process of turning from a liquid into vapor
transpiration: The process by which water is transferred from the land to the atmosphere by evaporation from the soil and other surfaces and by transpiration from plants
Infiltration: The process by which water on the ground surface enters the soil
Precipitation: Rain, snow, sleet, or hail that falls to the ground
Runoff: Part of the water cycle that flows over land as surface water instead of being absorbed into groundwater or evaporating
Water Distribution
Over 70% of Earth’s surface is covered by water, with oceans holding about 97% of all water on Earth, and freshwater making up only about 3%.
Of the freshwater that is available, most of it is trapped in glaciers and ice caps, with the rest found in groundwater, lakes, soil moisture, atmospheric moisture, rivers, and streams.
Water Properties
Strong hydrogen bonds hold water molecules together.
The temperature of water changes slowly due to its high specific heat capacity.
Water filters out harmful UV radiation in aquatic ecosystems.
Water has a high boiling point.
Water is a polar molecule
Capillary action: A result of hydrogen bonding, helps tree roots take up water, allowing trees to grow as large as they do.
universal solvent: dissolve many compounds.
Effects of Groundwater Depletion
Saltwater intrusion: The movement of saltwater into freshwater aquifers, which can lead to contamination.
1.8: Primary Productivity
The ultimate source of energy is the sun.
Photosynthesis: The plants remove carbon dioxide from the atmosphere and use light energy to produce glucose and oxygen:
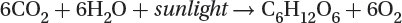
Plants capture light primarily through the green pigment chlorophyll, which is contained in organelles called chloroplasts.
Cellular Respiration
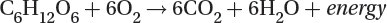
1.10:

Only 10% of a generation's biomass is transferred (10% rule or .10)
Second Law of Thermodynamics: States that as energy is transferred or transformed, more and more of it is wasted.
Ecosystem Productivity
Gross Primary Production (GPP)
Gross primary production (GPP): the rate at which solar energy is captured in sugar molecules during photosynthesis
Primary producers use some fixed energy for cellular respiration and tissue maintenance.
Net Primary Production (NPP)
Net primary production (NPP): The remaining fixed energy is the rate at which all the plants in an ecosystem produce net useful chemical energy.
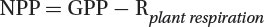
Open oceans collectively have the highest net primary productivity.
Chapter 2: The Living World (Biodiversity)
2.1: Introduction to Biodiversity
Biodiversity: the variety of life on planet Earth
helps the environment evolve to changes
Adaptation: The biological mechanism by which organisms adjust to new environments or to changes in their current environment.

Anthropogenic Activities Can Reduce Biodiversity
Burning Fossil Fuels
deforestations
Modern industrial agriculture: crop rotation, intercropping, polyculture
overfishing
use of pesticides
Genetically modified organism GMOs: decrease genetic variation
Population Bottleneck
Population Bottleneck: It is a large reduction in the size of a single population due to a catastrophic environmental event.
smaller population, less genetic diversity in the gene pool for future generations.
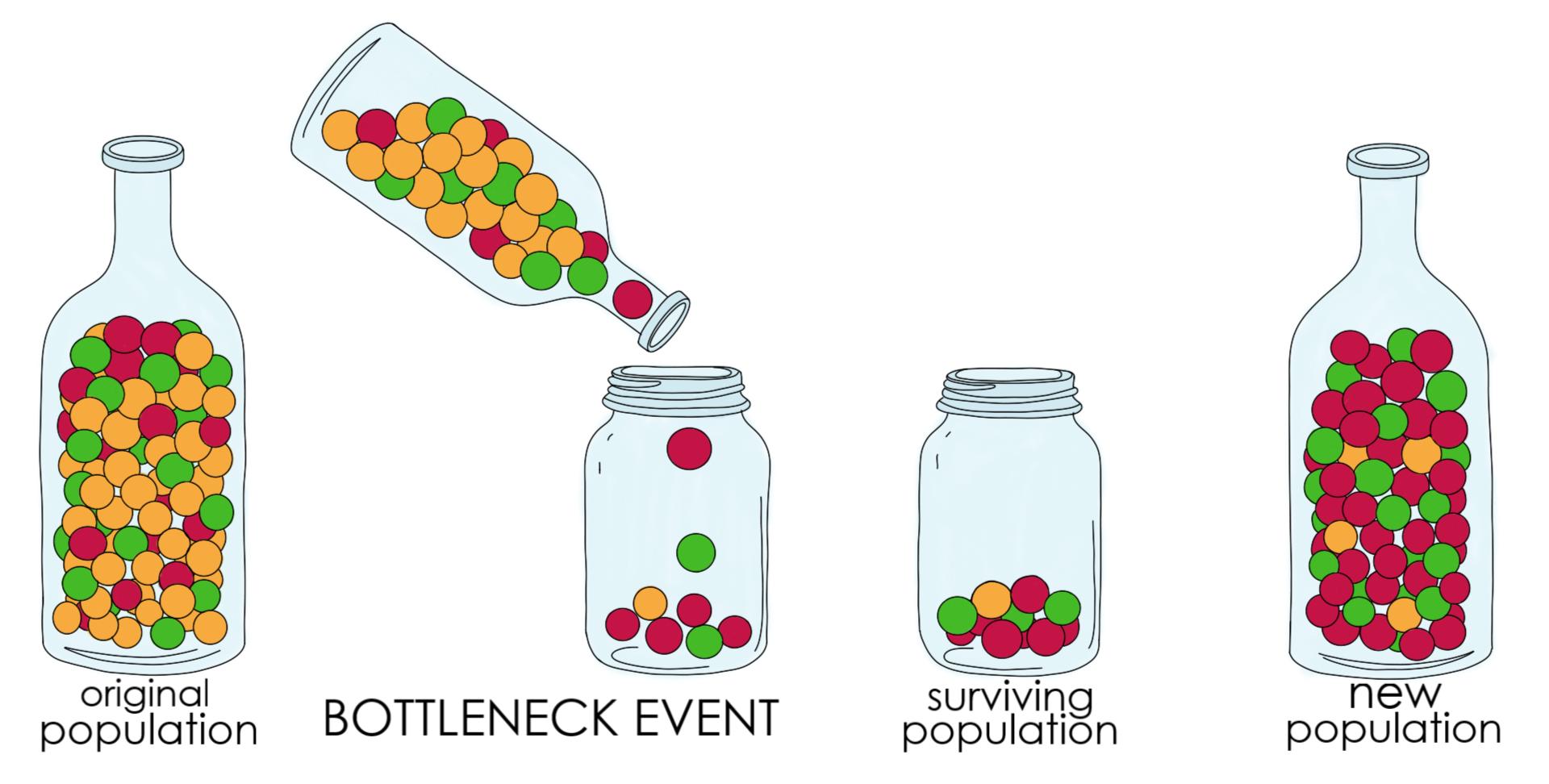
Loss of Habitat = Loss of Specialist Species
Generalist Species: Species that live in different types of environments and have varied diets.
Ex.: Raccoons or cockroaches
Specialist Species: These species require unique resources and limited diet; need a specific habitat in which to survive.
Ex.: Polar Bears: only durvive in extreme cold conditions
Species Richness: The number of different species (diversity) represented in an ecological community or region.
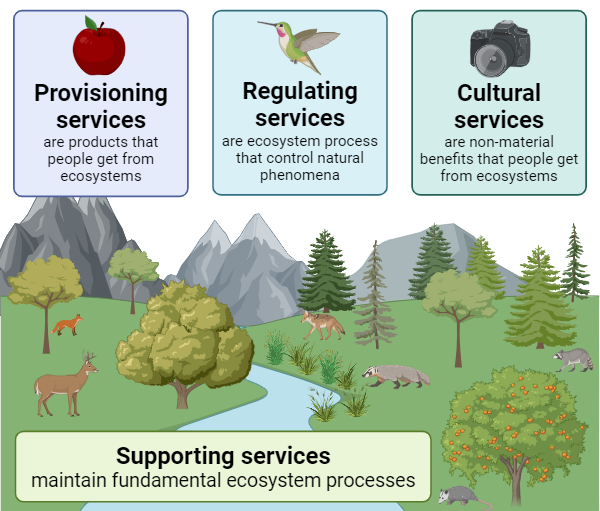
2.2: Ecosystem Services
2.3: Island Biogeography
Island closer to the mainland have more biodiversity since they are easier for migrating species.
Larger islands are bigger targets, so migrating species can find them more easily; more biodiversity; lower extinction rates.
2.4: Ecological Tolerance
biotic and abiotic factors; are regulated by the Law of Tolerance.
Law of Tolerance: It states that the existence, abundance, and distribution of species depend on the tolerance level of each species to change within its environment.
2.5: Natural Disruptions to Ecosystems
Ecosystem: A community of organisms that interact with each other and their environment and that can change over time.
Natural and sudden disruptions:
flooding
volcanic eruptions
wildfires
Primary vs. Secondary Succession
Primary succession: no soil; lifeless habitat
Secondary succession: after a major disturbance; soil
Ecological Succession in a Disturbed Ecosystem
Ecological disturbance: causing stress to the enviroment
Species richness: The number of different species represented in an ecological community.
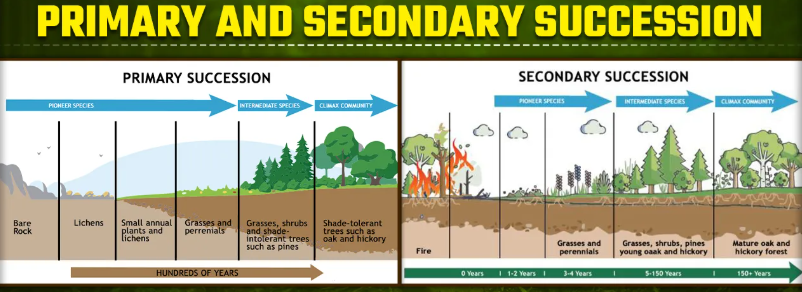
Species diversity and biodiversity increases with succession.
Species
Keystone Species : presence contributes to a diversity of life and whose extinction would lead to the extinction of other forms of life
Large influence on ecosystem
Pioneer Species: Earlier successional plants, generalists
Indicator Species: sensible to change
organisms whose presence, absence, or abundance can determine the health of an ecosystem.
Chapter 3: Populations
3.1: Generalists and Specialists Species
Generalist | Specialists |
Able to use a variety of environmental resources | Use specific set of resources. |
Adaptable to a wide range of environments | Less adaptable due to specialized needs |
Have a high level (range) of tolerance | Have a low level of tolerance |
Have an advantage when environmental conditions change | Easily affected when environmental conditions change |
Less likely to be extinct | More likely to become extinct |
Example: Human | Example: Panda |
3.2: K-Selected & R-Selected Species
r-Strategists | K-Strategists |
Not endangered | Most endangered |
Have many offspring and tend to overproduce | Have few offspring |
Low parental care | High parental care |
Mature rapidly | Mature slowly |
Population size limited by density-independent limiting factors, including climate, weather, natural disasters, and requirements for growth | Density-dependent limiting factors to population growth stem from intraspecific competition and include competition, predation, parasitism, and migration |
Short-lived | Long-lived |
Tend to be prey | Tend to be predator and prey |
Tend to be small | Tend to be larger |
Type III survivor curve | Type I or II survivor curve |
Wide fluctuations in population density | Population size stabilizes near the carrying capacity. |
Examples: most insects, algae, bacteria, rodents, and annual plants | Examples: humans, elephants, cacti, and sharks |
3.3: Survivorship Curve
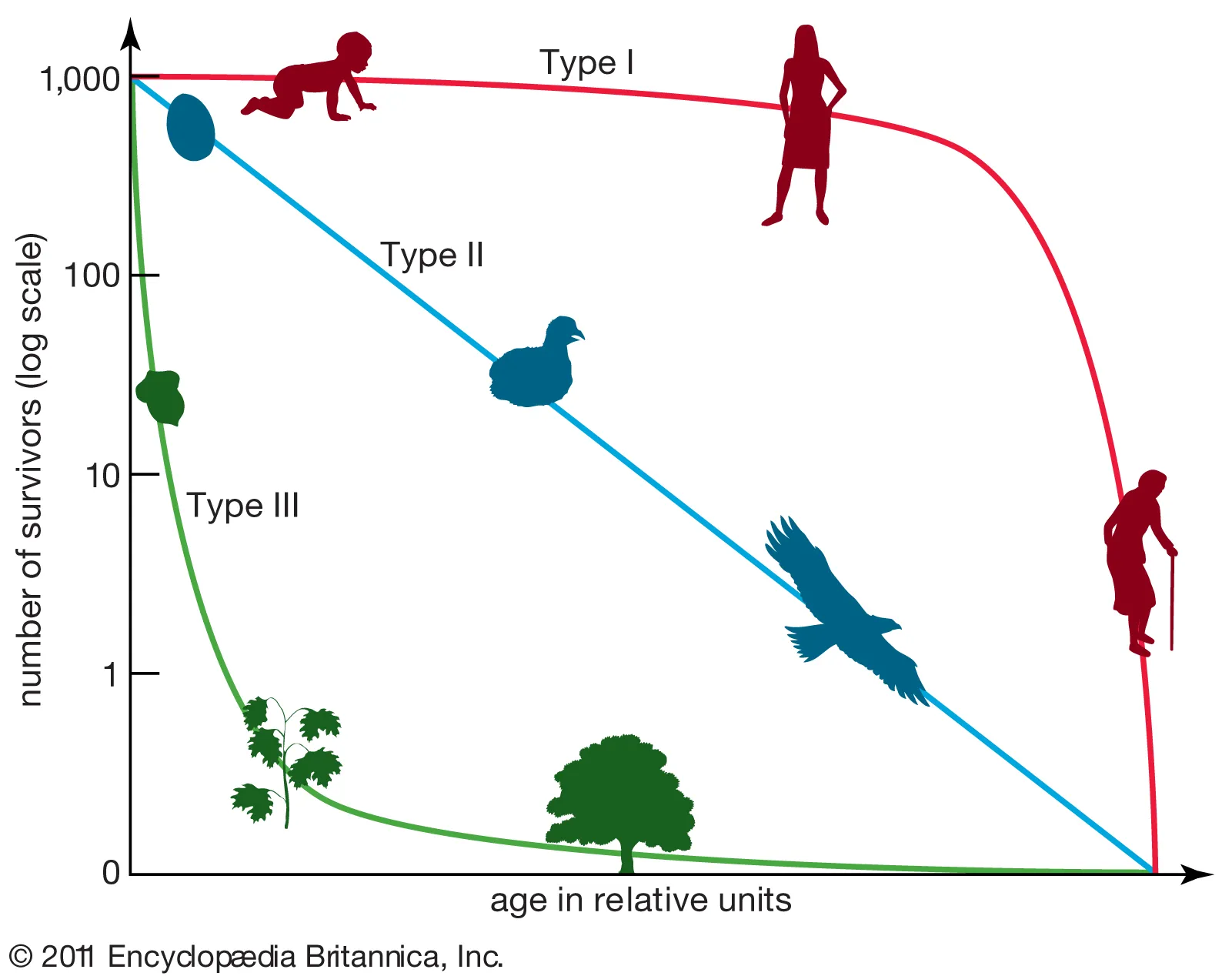
Survivorship curves: a graph showing the number or proportion of individuals surviving to each age for a given species or group
Type I - Late Loss
Low mortality at birth with a high probability of surviving to advanced age.
Examples: K selected species: humans and elephants.
Type II
Type III - Early Loss
great numbers of offspring and reproduce for most of their lifetime.
Die young
Examples: R-selected species
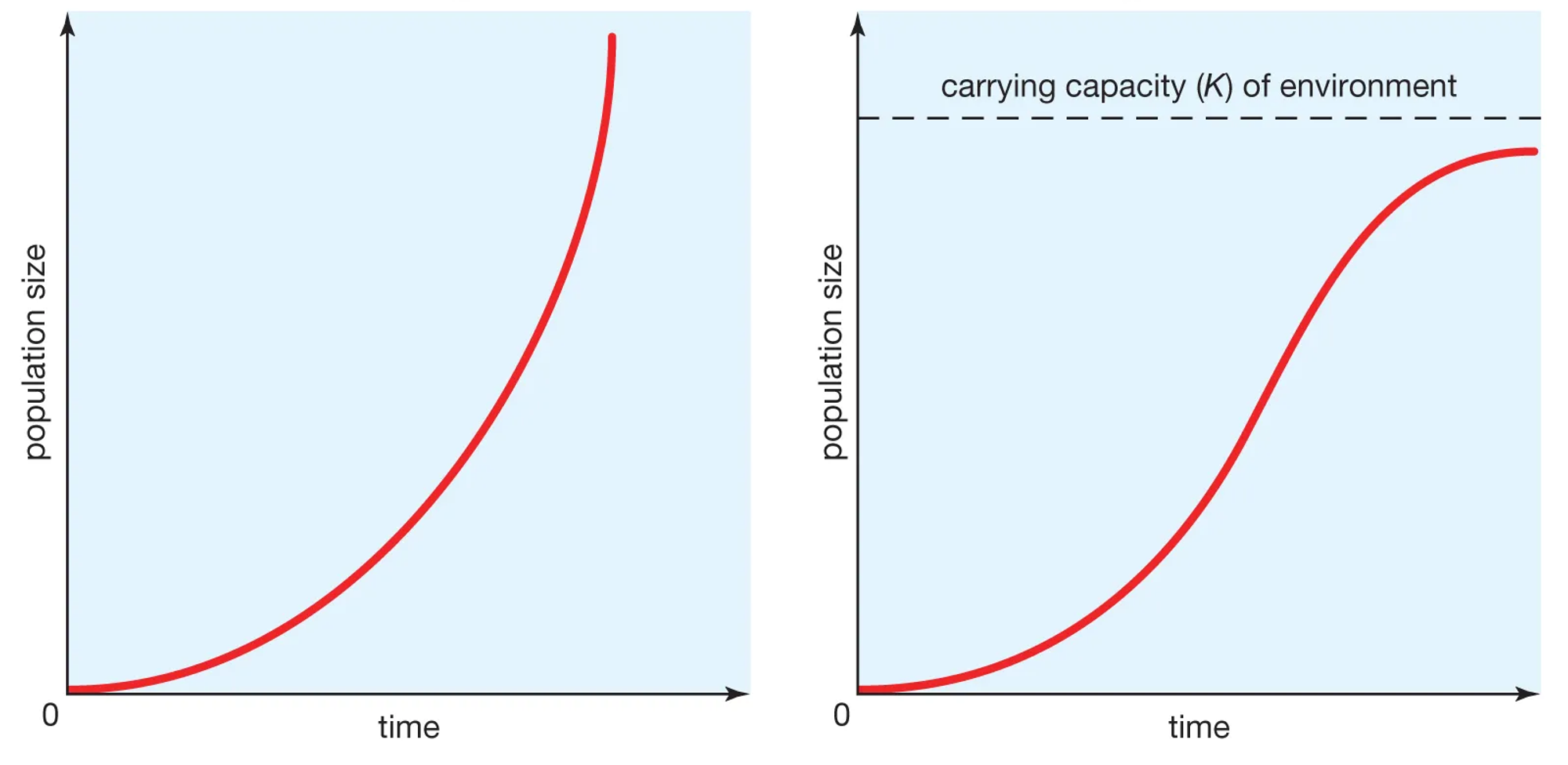
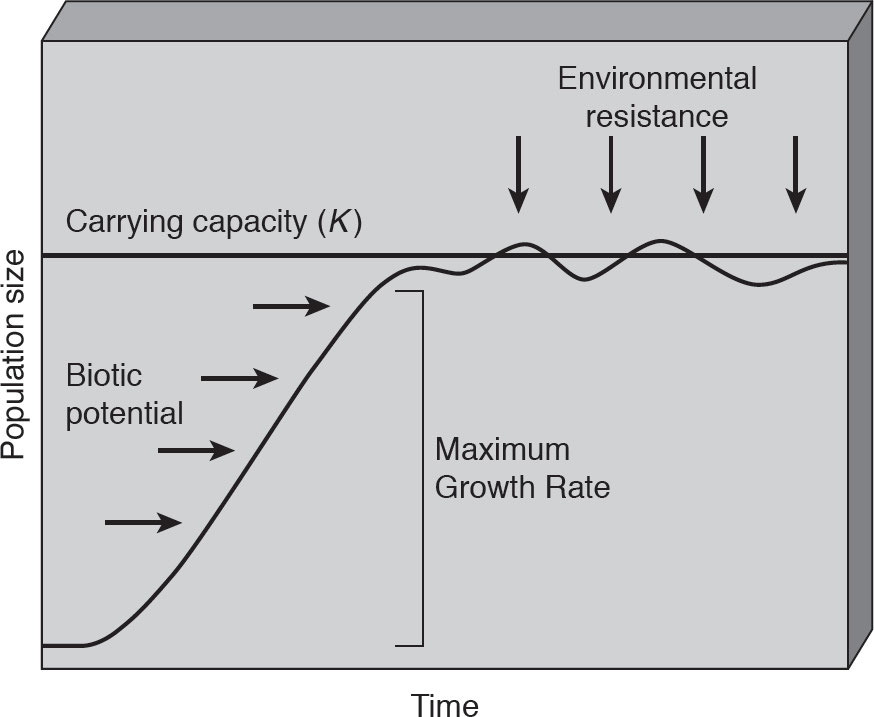
3.4: Carrying Capacity
Carrying capacity (K): It refers to the number of individuals that can be supported sustainably in a given area.
Factors that can affect K
Amount of sunlight available
Food availability
Nutr: k selected spcies ient levels in soil profiles
Oxygen content in aquatic ecosystems
Space
S-Curve:
J- Curve: exponential until they reach the K; optimal conditions
r -selected species
Biotic potential: The maximum reproductive capacity of an organism under optimum environmental conditions.
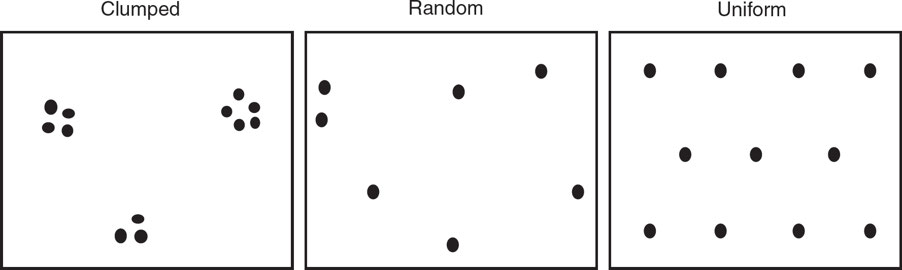
Population Dispersal Patterns
Clumped: patches; Some areas within a habitat are dense with organisms, while other areas contain few members.
impacted by density population factors
Random: Occurs in habitats where environmental conditions and resources are consistent
Individuals are distributed randomly;
Uniform: Space is maximized between individuals to minimize competition.
Feedback Loops
Positive feedback loops stimulate change; responsible for sudden or rapid changes within ecosystems.
Negative feedback loops often provide stability.
Limiting Factors
Density-dependent limiting factors: These are factors whose effects on the size or growth of the population vary with the density of the population.
Density-independent factors: These are factors that limit the size of a population, and their effects are not dependent on the number of individuals in the population.
Rule of 70
To find how long it takes for a population to double in size we can use the following formula: dt= 70/r
Populations cannot double forever due to carryin capapcity
The larger the growth rate (r), the faster the doubling time.
Important Population Formulas
Birth Rate (%) = [(total births/total population)] × 100
Crude Birth Rate (CBR) = [(b ÷ p) × 1,000]
Death Rate (%) = [(total deaths/total population)]× 100
Crude Death Rate (CDR) = [(d ÷ p) × 1,000]
Doubling Time = 70/% growth rate
Emigration = number leaving a population
Global Population Growth Rate (%) = [(CBR – CDR)]/10
Immigration = number entering a population
National Population Growth Rate (%) = [(CBR + immigration) – (CDR + emigration)]/10
Percent Rate of Change = [(new # - old #)/old #] × 100
Population Density = total population size/total area
Population Growth Rate (%) =
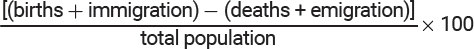
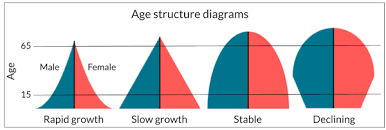
3.6: Age-Structure Diagrams
Age-structure diagrams: These are determined by birth rate, generation time, death rate, and sex ratios.
Pyramid-shaped age-structure diagram:
high birth rates
majority of the population is in the reproductive age group
High total Fertility Rate
High death rate
low growth rate
agricultural society
High birth rates replace high mortality, resulting in low population growth.
Bell shape age-structure diagram:
pre-reproductive and reproductive age groups are more nearly equal
industrializing to industrialized society
post-reproductive group being smallest due to mortality.
stable populations
Population growth is zero when birth and death rates are equal.
Urn-Shaped age-structure diagram:
pre-reproductive group is smallest
post-reproductive group is large
birth rate: low; below the death rate → declining populations
Post-industrialized society
sub-replacement fertility: how many kids it takes to replace parents (2)
3.7: Total Fertility Rate
Total fertility rate (TFR): number of births;
The average number of children that each woman will have
Declines in fertility rates:
As developing countries transition to developed countries, there is greater access to primary healthcare and family-planning services.
Female educational opportunities are increasing.
postponing marriage until their careers are established.
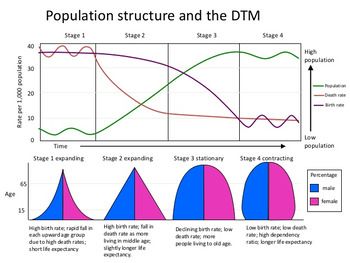
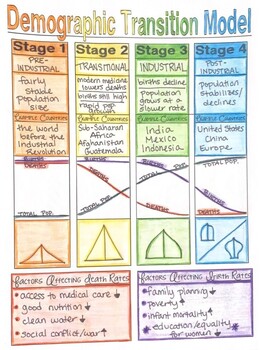
3.9: Demographic Transition
Demographic transition: It is the transition from high birth and death rates to lower birth and death rates as a country or region develops from a pre-industrial to an industrialized economic system.
Chapter 4: Earth’s Systems and Resources
4.1: Plate Tectonics
Plate tectonics: the understanding that the top layer or lithosphere of the Earth's crust is divided into many large rocky plates
Seafloor Spreading Theory
the geologic process that takes place when tectonic plates diverge, resulting in the creation of new ocean floors
The lithosphere is the solid, outer part of the Earth and is broken into huge sections called plates, which are slowly moving.
When one plate moves beneath another (subduction) or when two plates converge, it can result in earthquakes and volcanoes.
Subduction zones: These are areas on Earth where two tectonic plates meet and move toward each other, with one sliding underneath the other and moving down into the mantle.
Types of Boundaries
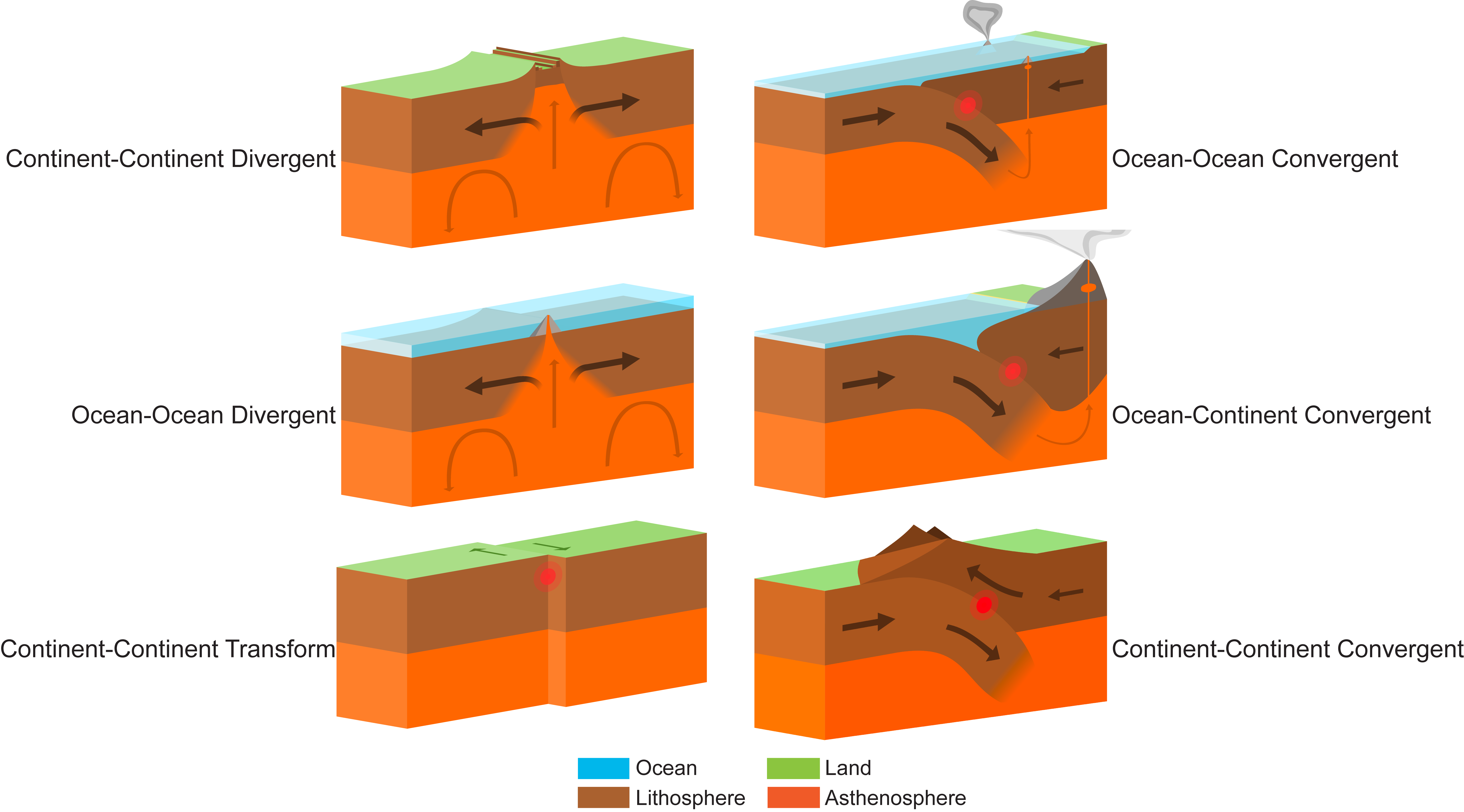
Convergent Boundaries: two plates slide toward each other.
Volcanic activity
mountains
island arc: a curved chain of volcanic islands rising from the deep seafloor and near a continent.
orogonic belt: if two plates collide and compress
a subduction zone: where one plate moves underneath the other
a denser oceanic plate subducts a less dense continental plate
Divergent Boundaries: two plates slide apart from each other; diverge
fault zones
Sea floor spreading
create new oceanic crust
ridges
Transform boundaries: plates slide past each other in opposite directions.
The friction and stress buildup from the sliding plates frequently causes earthquakes
Example: The San Andreas fault.
4.2: Soil Formation and Erosion
Soil
Surface Litter: Leaves and partially decomposed organic debris.
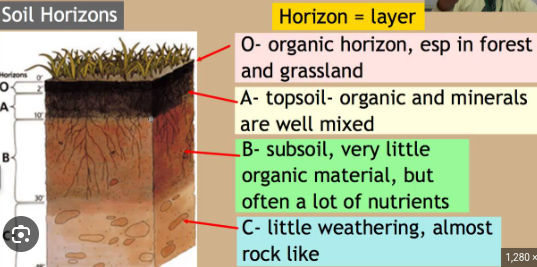
Soils develop in response to the following factors:
Climate
Living organisms: Include the nitrogen-fixing bacteria Rhizobium, insects.
earthworms: help mix organic materials in soil
Parent material: Refers to the rock and minerals from which the soil derives.
Topography: physical characteristics of the location.
pH: It is the measure of how acidic or basic soil is.
Pore Size: Describes the space between soil particles
Permeability: The measure of the capacity of the soil to allow water and oxygen to pass through it.
Low permeability can lead to soil salinization.
Soil Erosion
Soil erosion: the movement of weathered rock and/or soil components from one place to another caused by flowing water, wind, and human activity.
It decreases the soil’s water-holding capacity, destroys the soil profile, and increases soil compaction.
Poor agricultural techniques that lead to soil erosion includes:
Monoculture
Overgrazing
Rock Types
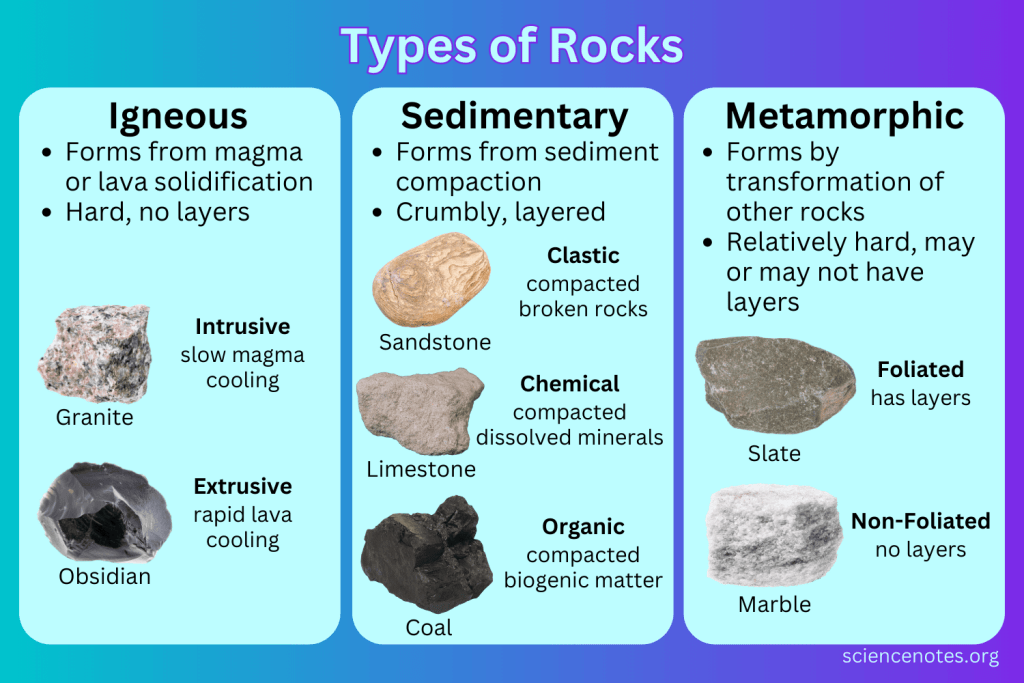
Igneous Rocks: formed by cooling
Igneous rocks are broken down by weathering and water transport.
Metamorphic Rocks: formed by intense heat and pressure (ex: diamond, marble)
Sedimentary: These are formed by the piling and cementing of various materials over time (ex: Fossils form)
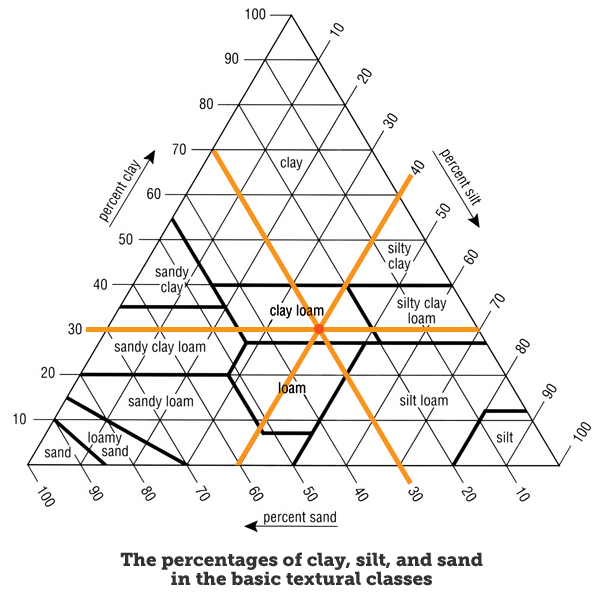
4.3: Soil Composition and Properties
Soil Components
Sand
big particles
High permeability: Water flows through too quickly for most crops.
Good for crops and plants requiring low amounts of water.
Loam
About equal mixtures of clay, sand, silt
Rich in nutrients.
Holds water but does not become waterlogged. Particle size can vary.
Silt
very fine particles between the sizes of sand and clay.
Easily transported by water.
Clay
Very fine particles.
Compacts easily.
Low permeability to water; therefore, upper layers become waterlogged.
Humus: Top soil; It is the dark organic material that forms in soil when plant and animal matter decays

4.4: Earth’s Atmosphere
Atmosphere’s Current Composition
Nitrogen (N2) — 78%
Fundamental nutrient for living organisms.
Found in all organisms, primarily in amino acids and nucleic acids.
Deposits on Earth through nitrogen fixation and reactions
Returns to the atmosphere through combustion of biomass and denitrification.
Oxygen (O2) — 21%
Product in photosynthesis and reactant in cellular respiration.
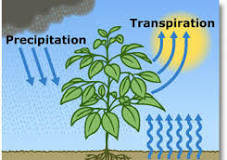
Water Vapor (H2O) — 0% to 4%
the transpiration of plants.
transpiration: the process of water movement through a plant and its evaporation from aerial parts, such as leaves, stems and flowers
Carbon Dioxide (CO2) < 1%
Produced during cellular respiration, the combustion of fossil fuels, and the decay of organic matter.
Required for photosynthesis
Major greenhouse gas contributing to global warming
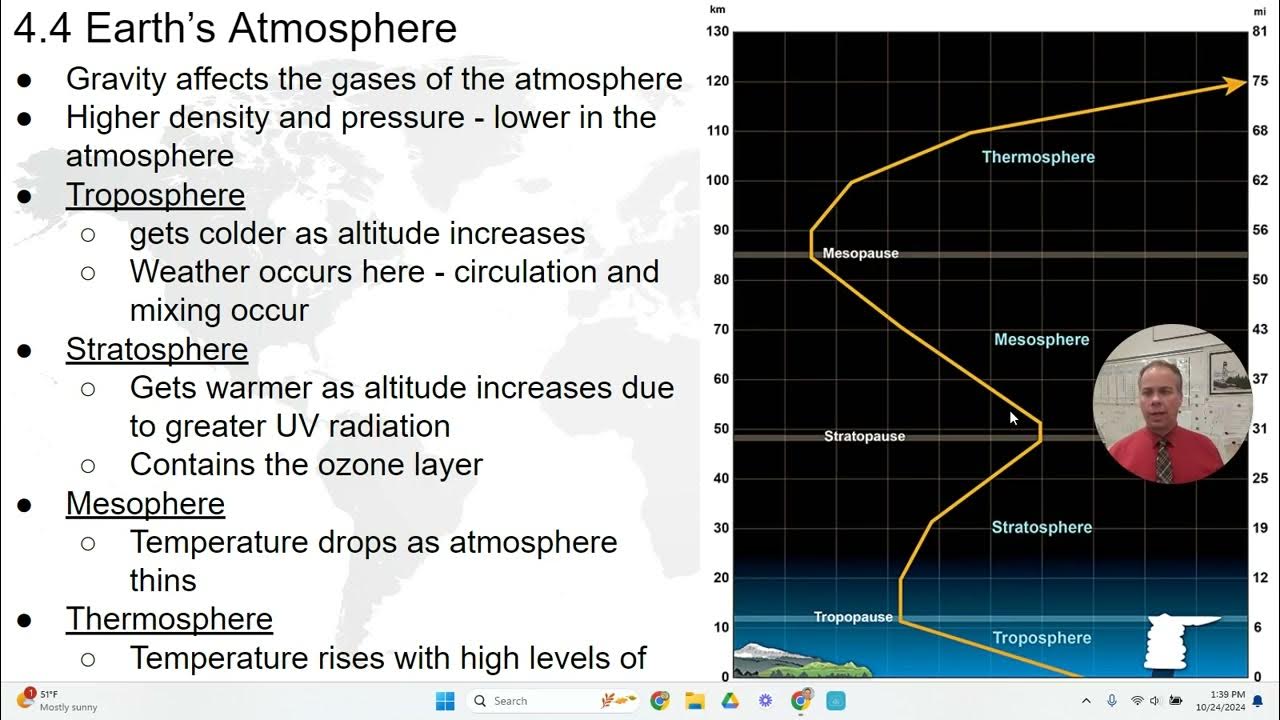
Atmosphere’s Structure
Troposphere: The lowest portion of Earth’s atmosphere
density/ pressure decreases as you increase height/ altitude
Stratosphere: ozone (O3) absorbs harmful UV radiation from the sun
NEED TO EDIT Below
Weather and Climate
Weather: It is caused by the movement or transfer of heat energy, which results from the unequal heating of Earth’s surface by the sun.
It describes whatever is currently happening outdoors.
It influences the following physical properties:
Air pressure
Air temperature
Humidity
Precipitation
Sunlight reaching Earth affected by cloud cover
Wind direction and speed
Climate: The average weather conditions prevailing in an area in general or over a long period.
The statistical description in terms of the mean and variability of relevant quantities over a period ranging from months to thousands or millions of years.
Convection: It is the primary way energy is transferred from hotter to colder regions in Earth’s atmosphere and is the primary determinant of weather patterns.
Warmer, more energetic air molecules move vertically and horizontally.
Air rises when it becomes warmer and less dense than the air above it, creating pressure differences that cause wind.
Heat Index (HI): The measure of how warm it feels when factoring in relative humidity.
Climate and Factors that Influence it
Air Mass: A large body of air that has similar temperature and moisture content.
These can be categorized as equatorial, tropical, polar, Arctic, continental, or maritime.
Albedo: An expression of the ability of surfaces to reflect sunlight.
Materials like ocean water have low albedo, whereas landmasses have moderate albedo.
Snow and ice have the highest albedo.
Altitude: The distance above sea level.
Carbon Cycle: The process in which carbon atoms continually travel from the atmosphere to the Earth and then back into the atmosphere.
Distance to Oceans: Oceans are thermally more stable than landmasses; the specific heat of water is five times greater than that of air.
Because of this, changes in temperature are more extreme in the middle of the continents than on the coasts.
Fronts: When two different air masses meet, the boundary between them forms a “front.”
The air masses can vary in temperature, dew point and wind direction.
Cold Front: The leading edge of an advancing mass of cold air and is associated with thunderhead clouds, high surface winds, and thunderstorms.
Warm Front: The boundary between an advancing warm air mass and the cooler one it is replacing.
Stationary Front: A pair of air masses, neither of which is strong enough to replace the other, that tend to remain in essentially the same area for extended periods of time.
Greenhouse Effect: Without this effect, Earth would be cold and inhospitable.
The most important greenhouse gases are water vapor (H2O), carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O).
If taken too far, however, Earth could evolve into a hothouse.
Heat: Climate is influenced by how heat energy is exchanged between air over the oceans and the air over land.
Human Activity and Climate: Climate can also be influenced by human activity.
Increased pollution alone tends to increase the amount of rainfall in urban areas by as much as 10% when compared with undeveloped areas.
Climate is also influenced by urbanization and deforestation.
Latitude and Location
Latitude: The measurement of the distance of a location on Earth from the equator.
The farther away from the equator, the less sunlight is available.
At the poles, the sun’s rays strike Earth at an acute angle, which spreads the heat over a larger area.
Climate is influenced by the location of high and low air pressure zones and where landmasses are distributed.
Moisture Content of Air: It is a primary determinant of plant growth and distribution and is a major determinant of biome type.
Pollution: Greenhouse gases are emitted from both natural sources and anthropogenic sources.
Rotation: Daily temperature cycles are primarily influenced by Earth’s rotation on its axis.
At night, heat escapes from Earth’s surface, and daily minimum temperatures occur just before sunrise.
Volcanoes
Sulfur-rich volcanic eruptions: It can eject material into the stratosphere, potentially causing tropospheric cooling and stratospheric warming.
Volcanic aerosols: These exist in the atmosphere for an average of one to three years.
Volcanic aerosols injected into the stratosphere can also provide surfaces for ozone-destroying reactions.
4.5: Global Wind Patterns
Land and Sea Breezes
Land Breeze: It occurs during relatively calm, clear nights when the land cools down faster than the sea, resulting in the air above the land becoming denser than the air over the sea.
Sea Breeze: It occurs during relatively calm, sunny days, the land warms up faster than the sea, causing the air above it to become less dense.
Atmospheric Circulation-Pressure
Air closer to Earth's surface is warmer and rises due to Earth's rotation on its axis, revolution around the sun, and tilt.
Cooler, denser, higher-elevation air sinks, causing convection and winds.
Low-pressure weather systems have lower pressure at their centers than elsewhere.
Where winds meet low pressure, air rises.
Air rises, condensing water vapor into clouds and precipitation.
High-pressure weather systems: They have higher pressure at their center than around them, so winds blow away from them.
They blow clockwise north of the equator and counterclockwise south of it, with air from higher in the atmosphere sinking down to fill the gaps left by outward-blowing air.
Cool, dense air descends toward Earth's surface and warms in high-pressure masses, which are usually associated with fair weather.
Trade Winds: These are the prevailing pattern of easterly surface winds found in the tropics near Earth’s equator, within the troposphere or lower portion of Earth’s atmosphere.
It have been used by captains of sailing ships to cross the world’s oceans.
Wind Speed: It is determined by pressure differences between air masses.
The greater the pressure difference is, the greater the wind speed.
Wind Direction: It is based on the direction from which wind originated.
Easterly: Wind coming from the east.
Westerly: Wind coming from the west.
Coriolis Effect: A phenomenon wherein earth’s rotation on its axis causes winds to not travel straight, which causes prevailing winds in the Northern Hemisphere to spiral clockwise out from high-pressure areas and spiral counterclockwise toward low-pressure areas.
Hadley Air Circulation
Air heated near the equator rises and spreads out north and south.
After cooling in the upper atmosphere, the air sinks back to Earth’s surface within the subtropical climate zone.
Surface air from subtropical regions returns toward the equator to replace the rising air.
The equatorial regions of the Hadley cells are characterized by high humidity, high clouds, and heavy rains.
Subtropical regions of the Hadley cell are characterized by low relative humidity, little cloud formation, high ocean evaporation due to the low humidity, and many of the world’s deserts.
The climate is characterized by warm to hot summers and mild winters. The tropical wet and dry (or savanna) climate has a dry season more than two months long.
Ferrel Air Circulation Cells
Ferrel cells develop between 30° and 60° north and south latitudes.
The descending winds of the Hadley cells diverge as moist tropical air moves toward the poles in winds known as the westerlies.
Mid-latitude climates can have severe winters and cool summers due to mid-latitude cyclone patterns.
Defined seasons are the rule, with strong annual cycles of temperature and precipitation.
Climates of the middle latitudes have a distinct winter season.
Polar Air Circulation Cells
Polar cells originate as icy-cold, dry, dense air that descends from the troposphere to the ground.
This air meets with the warm tropical air from the mid-latitudes and then returns to the poles, cooling and then sinking.
Sinking air suppresses precipitation. As a result, the polar regions are deserts.
Very little water exists in this area because it is tied up in the frozen state as ice.
The amount of snowfall per year is relatively small.
Polar Vortex
Polar Vortex: A low-pressure zone embedded in a large mass of very cold air that lies atop both poles.
The bases of the two polar vortices are located in the middle and upper troposphere and extend into the stratosphere.
Due to the equator-pole temperature difference, these cold, low-pressure areas strengthen in winter and weaken in summer.
There is also a relationship between the chemistry of the Antarctic polar vortex and severe ozone depletion.
Hurricanes
Hurricanes, cyclones, and typhoons are all the same weather phenomenon.
Hurricanes: Term used in the Atlantic and Northeast Pacific.
Cyclones: Term used in South Pacific and Indian Ocean.
Typhoons: Term used in Northwest Pacific.
Hurricanes begin over warm oceans in areas where the trade winds converge.
A subtropical high-pressure zone creates hot daytime temperatures with low humidity that allow for large amounts of evaporation, with the Coriolis effect initiating the cyclonic flow.
Hurricane development requires tropical ocean thunderstorms and cyclonic circulation that starts to rotate them.
This cyclonic circulation allows them to pick up moisture and latent heat energy from the ocean.
In the center of the hurricane is the eye, an area of descending air and low pressure.
Storm Surge: A rise in sea level that occurs during tropical cyclones, typhoons, or hurricanes.
These storms produce strong winds that push the seawater toward the shore, which often leads to flooding.
Tornadoes
Tornadoes: These are wirling masses of air with wind speeds close to 300 miles per hour (485 kph).
The center of the tornado is an area of low pressure.
Formation of Tornadoes
Thunderstorm or hailstorm creates strong winds.
The strong winds begin to rotate (due to updrafts and downdrafts) and form a column of spinning air called a mesocyclone.
The mesocyclone meets warm air moving up and cold air moving down and creates a funnel.
The funnel, made up of dust, air, and debris, reaches the ground, and a tornado is formed.
Monsoons
Monsoons: These are strong, often violent winds that change direction with the season.
Monsoon winds: These blow from cold to warm regions because cold air takes up more space than warm air.
Monsoons blow from the land toward the sea in winter and from the sea toward land in the summer.
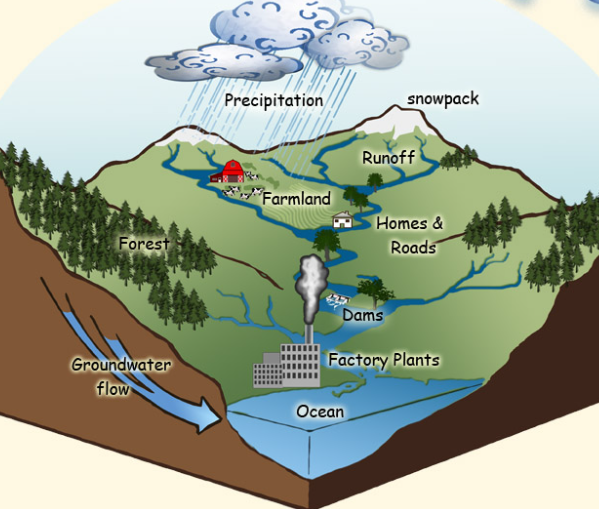
4.6: Watershed
Watershed: think like a natural funnel
4.7: Solar Radiation and Earth’s Seasons
Angle of Sunlight
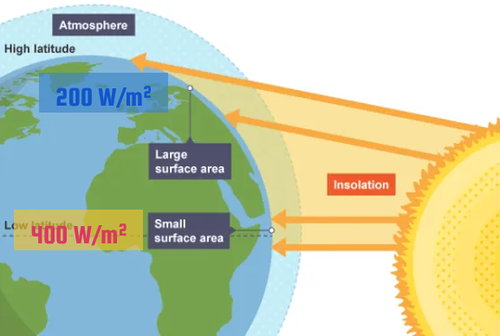
The amount of heat energy received at any location on Earth is a direct effect of the angle of the sunlight reaching the Earth’s surface.
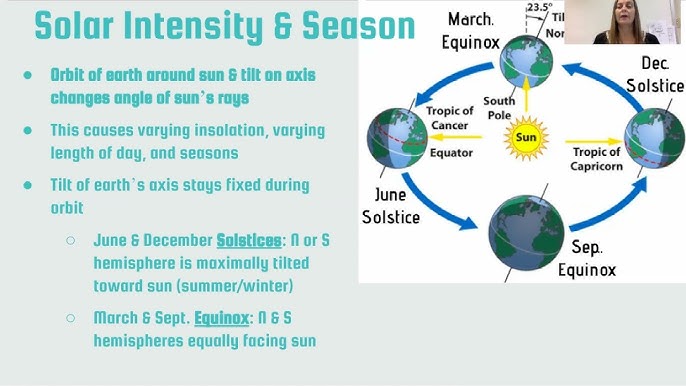
The angle at which sunlight strikes Earth varies due to Earth’s orbit around the sun and its rotation around its tilted axis.
Seasonal changes
Earth’s axis tilt: (23.5°)
Sunlight shining on Earth at a lower angle spreads its energy over a larger area, making it weaker than if the sun were higher overhead.
4.8: Earth’s Geography and Climate
Bodies of Water Moderate Climate and Regulate Precipitation
Over 70% of the Earth’s surface is covered in water.
Oceans and lakes store solar radiation (heat), and as the water heats up it adds moisture to the air above it
beginning a process that drives the major air currents around the world.
Large water bodies also tend to stabilize the climate of adjacent land masses by absorbing extra heat during warm periods and releasing it during cooler periods.
Warm, moist ocean air is a driving force for precipitation patterns around the world as it is carried over cooler land masses.
Higher Elevations Have Cooler Climates
Climates become cooler and the cold season lasts longer as elevation increases.
Higher elevations have lower air pressure due in part to there being fewer atoms and molecules per unit of air and, thus, cooler temperatures.
Many high-altitude plains are technically deserts because they are on the downwind (leeward) side of a mountain range or continental mass.
Latitude: A measure of distance either north or south from the equator.
Tropic of Cancer: The northernmost latitude reached by the overhead sun.
Tropic of Capricorn: The southernmost latitude reached by the overhead sun.
Mountains Affect Air Flow
Mountain ranges: These are barriers to the smooth movement of air currents across continents.
When an air mass hits mountains, it slows down and cools because the air is forced up into cooler parts of the atmosphere to move over the mountains.
The cooled air can't hold as much water anymore, so it rains on the side of the mountain range that faces the wind.
The mountain range's leeward side is drier than the windward side because air on this side has less moisture.
Rain Shadow Effect: The drier situation which is directly responsible for the plants that grow there, which in turn affects the animals that live there.
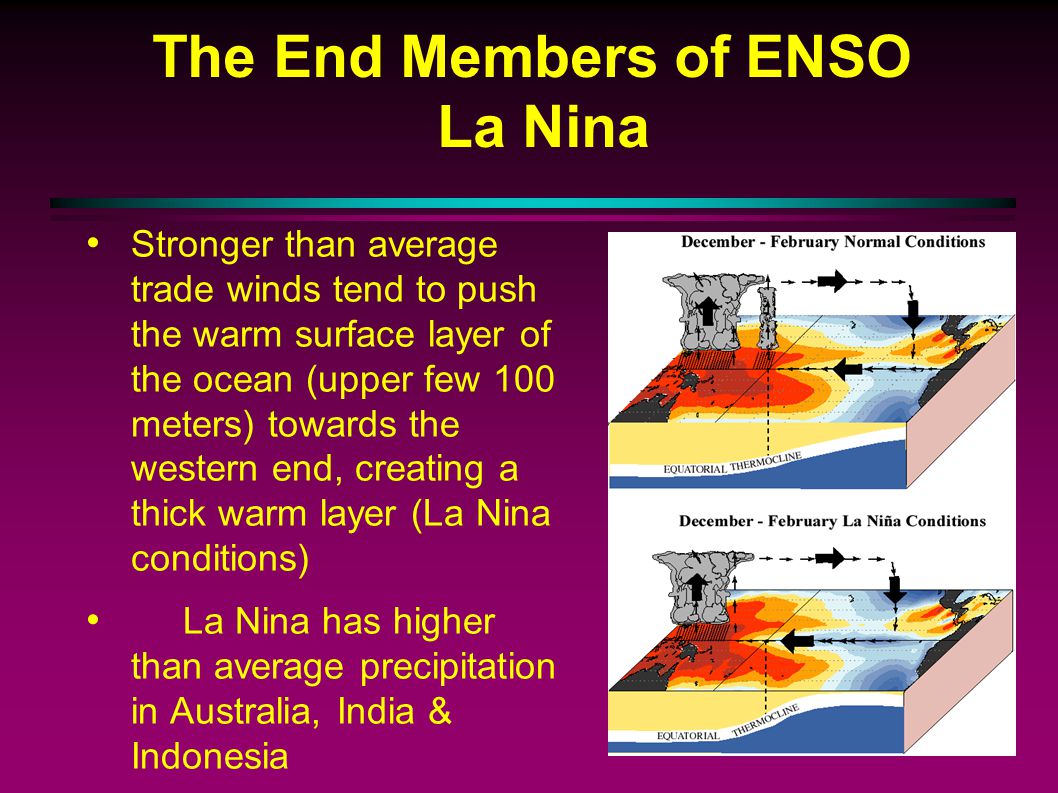
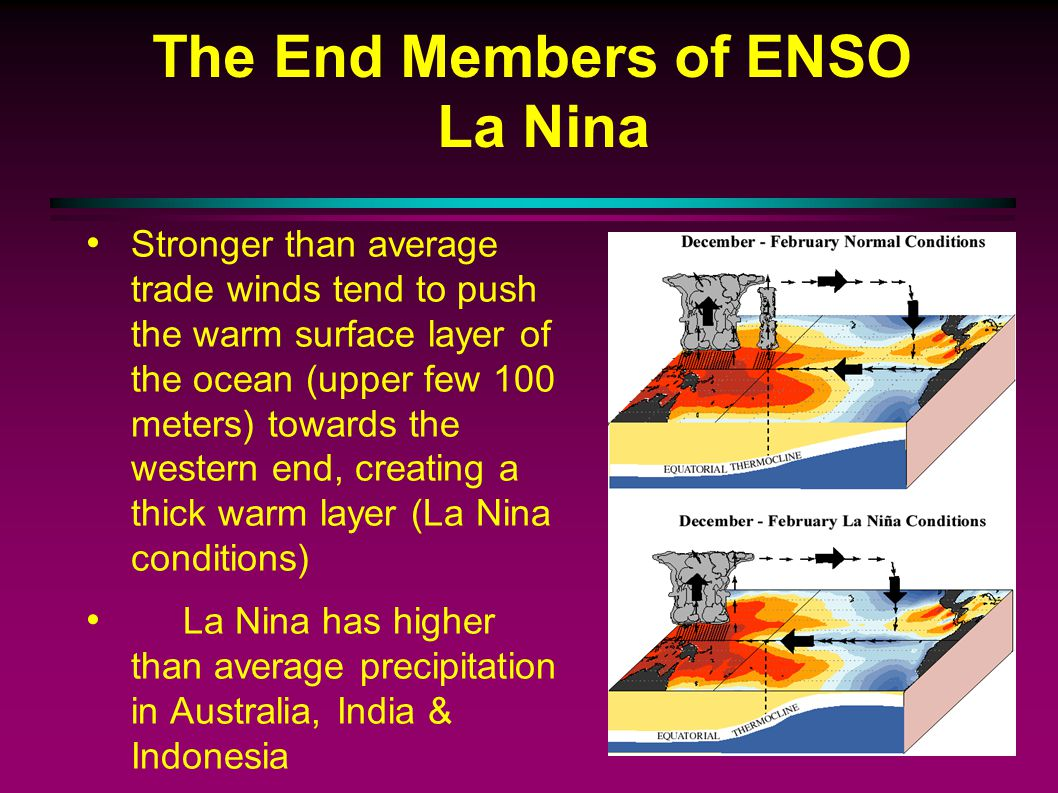
4.9: El Niño and La Niña
La Nada (Normal Conditions)
During normal conditions, easterly trade winds move water and air toward the west.
The ocean is generally around 24 inches (60 cm) higher in the western Pacific, and the water there is about 14°F warmer.
The trade winds, in piling up water in the western Pacific, make a deep warm layer in the west that pushes the thermocline down while it rises in the east.
Upwelling: It occurs when prevailing winds, produced through the Coriolis effect and moving clockwise in the Northern Hemisphere, push warmer, nutrient-poor surface waters away from the coastline
It is caused by winds pulling nutrient-rich water from below, increasing fishing stocks in this shallow eastern thermocline (90 feet or 30 m).
El Niño (Warm Phase)
Air pressure patterns reverse direction, causing trade winds to decrease in strength.
This causes the normal flow of water away from western South America to decrease “pile up.”
As a result, the thermocline off western South America becomes deeper and there is a decrease in the upwelling of nutrients, which causes extensive fish kills.
A band of warmer-than-average ocean water temperatures develops off the Pacific coast of South America.
Effects are strongest during the Northern Hemisphere winter because ocean temperatures worldwide are at their warmest.
Increased ocean warmth enhances convection, which then alters the jet stream
La Niña (Cool Phase)
Trade winds that blow west across the tropical Pacific are stronger than normal.
This then results in an increase in the upwelling off of South America.
This then results in cooler-than-normal sea surface temperatures off of South America.
This then results in wetter-than-normal conditions across the Pacific Northwest, and both drier- and warmer-than-normal conditions in the southern United States.
This then results in an increase in the number of hurricanes.
The southeastern US has warmer winters and the northwest cooler ones, while India and southeast Asia have heavier monsoons.
Environmental Effects of ENSO Weather Patterns
Warmer or cooler ocean temperatures
A decrease in upwelling, resulting in die-offs.
A negative impact on coral reefs.
Animal migration patterns may become disrupted.
Changes in weather patterns may increase insect-borne diseases.
Marine food webs and biodiversity may be disrupted by species that cannot tolerate warmer or cooler water temperatures.
Global warming decreases as warmer ocean water can hold less CO2.
Hurricanes and tornadoes may become stronger and more frequent.
Ocean currents and glacial melting may change with warmer ocean temperatures.
Increase or decrease in the amount of normal rainfall
Reduced rainfall may increase food competition, agricultural output, migration patterns, starvation, species die-offs, forest fires, and water shortages.
An increase in rainfall may result in an increase in flooding, soil erosion, and leaching of nutrients from the soil.
El Niño and La Niña
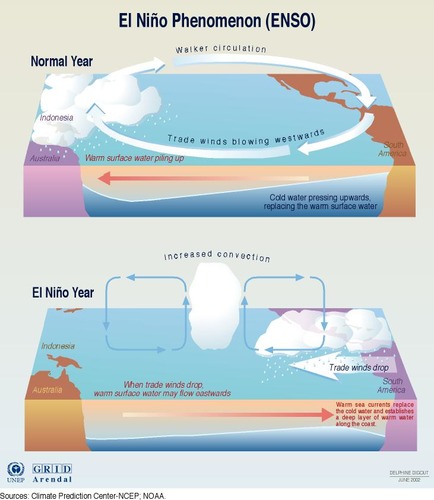
no cold rich nutrient water in South America; instead warm water is along the coast
La Niña: is a climate pattern that occurs when the surface waters of the Pacific Ocean become unusually cool, leading to changes in weather patterns around the world.
Chapter 5: Land and Water Use
5.1: The Tragedy of the Commons
Garrett Hardin wrote “The Tragedy of the Commons” in 1968.
The essay parallels what is happening worldwide in regards to resource depletion and pollution.
The seas, air, water, animals, and minerals are all “the commons” and are for humans to use, but those who exploit them become rich.
The following environmental issues echo "The Tragedy of the Commons" sustainability issues:
Air pollution
Burning of fossil fuels and consequential global warming
Frontier logging of old-growth forests and the practice of “slash and burn”
Habitat destruction and poaching
Over-extraction of groundwater and wastewater due to excessive irrigation
Overfishing
Overpopulation
Limits to “The Tragedy of the Commons” include the following:
Dividing a "commons" into privately owned parcels fragments its policies.
Different standards and practices on one parcel may or may not affect all parcels. Environmental decisions are long-term, while economic decisions are short-term.
Investors would be encouraged to pay a short-term price for a long-term gain by including discount rates in resource valuation.
Market pressure affects privately owned land.
Controlling some "commons" is easier than others. Air and the open oceans are harder to control than land, lakes, rangeland, deserts, and forests.
5.2: Clear-Cutting
Clear-cutting: It occurs is when all of the trees in an area are cut at the same time.
Environmental impacts of clear-cutting include the following:
Habitat loss reduces biodiversity.
Allows sunlight to reach the ground, making it warmer and drier, unsuitable for many forest plants.
Temporary wood availability followed by long periods without wood Reduction in long-term and short-term carbon sinks, which increases atmospheric CO2
Runoff increases soil erosion.
Edge Effect: It refers to how the local environment changes along some type of boundary or edge.
Forest edges: These are created when trees are harvested, particularly when they are clear-cut.
Tree canopies: It provide the ground below with shade and maintain a cooler and moister environment below.
Deforestation: It is the conversion of forested areas to non-forested areas, which are then used for grain and grass fields mining, petroleum extraction, fuel wood cutting, commercial logging, tree plantations, or urban development.
Impacts of deforestation include the following:
Runoff into aquatic ecosystems, climate change, and erosion decrease soil fertility.
Without shade, forest soils dry out quickly.
Degrading environment(s) with decreased biodiversity and ecological services.
Forests house 80% of land animals and plants.
Increasing habitat fragmentation and CO2 emissions from burning and tree decay.
Reducing migratory bird and butterfly habitats
Endangering niche-specialized species.
Deforestation Mitigation
Adopting uneven-aged forest management practices.
Educating farmers about sustainable forest practices and their advantages.
Monitoring and enforcing timber-harvesting laws.
Growing timber on longer rotations.
Reducing fragmentation in remaining large forests.
Reducing road building in forests.
Reducing or eliminating the practice of clear-cutting.
Relying on more sustainable tree-cutting methods.
5.3: The Agricultural and Green Revolutions
Agricultural Revolutions
First Agricultural Revolution (2000+ B.C.E.)
People went from hunting and gathering to the domestication of plants and animals, which allowed people to settle in areas and create cities.
Settled communities permitted people to observe and experiment with plants to learn how they grow and develop.
Second Agricultural Revolution (1700–1900 C.E.)
Occurred at the same time as the Industrial Revolution—mechanization had a major role in this revolution and changed the way people farmed.
Advances were made in breeding livestock.
Increased agricultural output made it possible to feed large, urban populations.
Methods of soil preparation, fertilization, crop care, and harvesting improved.
New banking and lending practices helped farmers afford new equipment and seed.
New crops came into Europe from trade with the Americas.
Railroads allowed distribution of products.
The invention of the seed drill allowed farmers to avoid wasting seeds and to plant in rows.
The invention of the tractor, combined with other farm machinery, improved efficiency on farms.
Third Agricultural Revolution (1900 C.E.–present)
Mechanization such as tractors and combines requires less labor and makes food prices more affordable.
Scientific farming methods such as biotechnology, genetic engineering, and the use of pesticides are now beginning to focus on more sustainable methods.
Green Revolutions
First Green Revolution (1940s–1980s)
The introduction of inorganic fertilizers, synthetic pesticides, new irrigation methods, and disease-resistant, high-yielding crop seeds.
Second Agricultural Revolution (1980s–Present)
In the mid-1980s, new engineering techniques and free-trade agreements involving food production property rights shaped agricultural policies and food production and distribution systems worldwide.
This revolution saw the development and spread of genetically modified organisms (GMOs)—animals, plants, and microorganisms—with genes that don't exist in nature.
BT corn and Golden Rice, modified with daffodil genes to produce more beta-carotene (converts to Vitamin A), are examples (corn modified with a bacterial insecticide gene that produces insect toxins within the cells of the corn).
5.4: Agricultural Practices
Agricultural productivity: It implies greater output with less input.
As farms become more efficient, they are able to produce more products at a lower cost, which tends to stabilize food prices and make more food available to more people, which is vital for developing countries.
Desertification: It is the conversion of marginal rangeland or cropland to a more desert-like land type.
Overgrazing: A plant is considered overgrazed when it is re-grazed before the roots recover, which can reduce root growth by up to 90%.
Fertilizers: These provide plants with the nutrients needed to grow healthy and strong.
Inorganic Fertilizers: A fertilizer mined from mineral deposits or manufactured from synthetic compounds.
Organic Fertilizers: Any Any fertilizer that originates from an organic source, such as bone meal, compost, fish extracts, manure, or seaweed.
Genetically modified foods: These are foods produced from organisms both animal and plant) that have had changes introduced into their DNA.
Genetic engineering techniques: These allow for the introduction of new traits as well as greater control over traits when compared to previous methods.
Rangelands: These are native grasslands, woodlands, wetlands, and deserts that are grazed by domestic livestock or wild animals.
These are managed through livestock grazing and prescribed fire rather than more intensive agricultural practices of seeding, irrigation, and the use of fertilizers.
Slash-and-Burn Agriculture: It is a widely used method of growing food or clearing land in which wild or forested land is clear-cut and any remaining vegetation is burned.
Soil Erosion: It is the movement of weathered rock or soil components from one place to another and is caused by flowing water, wind, and human activity.
Soil degradation: It is the decline in soil condition caused by its improper use or poor management, usually for agricultural, industrial, or urban purposes.
Desertification: Productive potential of arid or semiarid land falls by at least 10% due to human activity and/or climate change.
Salinization: Water that is not absorbed into the soil evaporates, leaving behind dissolved salts in topsoil.
Waterlogging: Saturation of soil with water, resulting in a rise in the water table.
Tillage: An agricultural method in which the surface is plowed and broken up to expose the soil, which is then smoothed and planted.
5.5: Irrigation Methods
Irrigation: The application of controlled amounts of water to plants at needed intervals and has been a necessary component of agriculture for over 5,000 years.
Ditch: Dug and seedlings are planted in rows.
The plantings are watered by placing canals or furrows in between the rows of plants.
Siphon tubes are used to move the water from the main ditch to the canals.
Drip: Water is delivered at the root zone of a plant through small tubes that drip water at a measured rate.
Flood: Water is pumped or brought to the fields and is allowed to flow along the ground among the crops.
Being simple and inexpensive, it is the method most widely used in less-developed countries.
Furrow (Channel): Small parallel channels are dug along the field length in the direction of the predominant slope.
Water is applied to the top of each furrow and flows down the field under gravity, infiltrating the ground more at the beginning and less at the end.
Spray: Uses overhead sprinklers, sprays or guns to spray water onto crops.
5.6: Pest-Control Methods
Pesticides: These can be used to control pests, but their use has drawbacks.
Integrated Pest Management (IPM): It is an ecologically based approach to control pests.
Types of Pesticides
Biological Pesticides: Living organisms used to control pests.
Carbamates: Also known as urethanes, affect the nervous system of pests, which results in the swelling of tissue in the pest.
Fumigants: These are used to sterilize soil and prevent pest infestation of stored grain.
Inorganic pesticides: These are broad-based pesticides that include arsenic, copper, lead, and mercury. They are highly toxic and accumulate in the environment.
Organic pesticides: These are natural poisons derived from plants such as tobacco or chrysanthemum.
Organophosphates: These are extremely toxic but remain in the environment for only a brief time.
Persistent Organic Pollutants (POPS)
Persistent organic pollutants (POPS): These organic compounds can pass through and accumulate in living organisms' fatty tissues because they don't break down chemically or biologically.
They also biomagnify food pyramids.
The Pesticide Treadmill
Pesticide resistance: It describes the decreased susceptibility of a pest population to a pesticide that was previously effective at controlling the pest.
Pest species: They evolve pesticide resistance via natural selection.
In response to resistance, farmers may increase pesticide quantities and/or the frequency of pesticide applications, which magnifies the problem.
Pesticide Treadmill: Also known as pest traps; farmers are forced to use more and more toxic chemicals to control pesticide-resistant insects and weeds.
Integrated Pest Management (IPM)
IPM: It is an ecological pest-control strategy that uses a combination of biological, chemical, and physical methods together or in succession and requires an understanding of the ecology and life cycle of pests.
Methods used in IPM include the following:
Construction of mechanical controls.
Developing genetically modified crops that are more pest-resistant.
Intercropping: A farming method that involves planting or growing more than one crop at the same time and on the same piece of land.
Natural insect predators
Planting pest-repellant crops
Polyculture: The simultaneous cultivation or raising of several crops or types of animals
Regular monitoring through visual inspection and traps followed by record keeping
Releasing sterilized insects
Rotating crops often to disrupt insect cycles
Using mulch to control weeds
Using pheromones or hormone interrupters
Using pyrethroids or naturally occurring microorganisms
When used effectively, IPM can reduce the following:
Bioaccumulation and biomagnification of pesticides
Pests’ becoming resistant to a particular pesticide
Genetic resistance: An inherited change in the genetic makeup of the pests that confers a selective survival advantage.
The destruction of beneficial and non-targeted organisms.
5.7: Meat Production Methods
Concentrated Animal Feeding Operations (CAFOs)
CAFO: It is an intensive animal feeding operation in which large numbers of animals are confined in feeding pens for over 45 days a year.
The large amounts of animal waste from CAFOs present a risk to water quality and aquatic ecosystems.
States with high concentrations of CAFOs experience on average 20 to 30 serious water-quality problems per year as a result of manure management issues.
Manure discharge from CAFOs can negatively impact water quality.
The two main contributors to water pollution caused by CAFOs are
soluble nitrogen compounds
phosphorus
Water pollution from CAFOs can affect both sources if one or the other is contaminated.
CAFOs release several types of gas emissions—ammonia, hydrogen sulfide, methane, and particulate matter.
The primary cause of gas emissions from CAFOs is the decomposition of animal manure being stored in large quantities.
5.8: Overfishing
Fishing is an important industry that is under pressure from growing demand and falling supply.
Marine life, including fisheries, as well as terrestrial life, depends upon primary producers.
Aquatic plants require sunlight and are therefore largely restricted to shallow coastal waters, which make up less than 10% of the world’s ocean area yet contain 90% of all marine species.
Aquaculture: Mariculture or fish farming. It includes the commercial growing of aquatic organisms for food and involves stocking, feeding, protecting from predators, and harvesting.
For aquaculture to be profitable, the species must be marketable, inexpensive to raise, efficient at converting feed into fish biomass, and disease resistant.
Methods to Manage Marine Fishing
Eliminate government subsidies for commercial fishing.
Increase the number of marine sanctuaries.
Prevent the importation of fish products from countries that do not adhere to sustainable fishing practices.
Require and enforce labeling of fish products that were raised or caught according to sustainable methods.
Require fishing licenses and open inspections, which limit the number and kind of fish caught per year, and trade sanctions should these limits be exceeded.
Methods to Restore Freshwater Fish Food Webs
Control erosion.
Control invasive species.
Create or restore fish passages.
Enforce laws that protect coastal estuaries and wetlands.
Plant native vegetation on stream banks.
5.9: Mining
Mining: Removing mineral resource from the ground.
Can involve underground mines, drilling, room-and-pillar mining, long-wall mining, open pit, dredging, contour strip mining, and mountaintop removal.
Surface Mining
Contour mining: Removing overburden from the seam in a pattern following the contours along a ridge or around a hillside.
Dredging: A method for mining below the water table and usually associated with gold mining.
Small dredges use suction or scoops to bring the mined material up from the bottom of a body of water.
In situ: Small holes are drilled into the Earth and toxic chemical solvents are injected to extract the resource.
Mountaintop removal: Removal of mountaintops to expose coal seams and disposing of associated mining overburden in adjacent “valley fills”
Open pit: Extracting rock or minerals from the Earth by their removal from an open pit when deposits of commercially useful ore or rocks are found near the surface
Strip mining: Exposes coal by removing the soil above each coal seam
Underground Mining
Blast: Uses explosives to break up the seam, after which the material is loaded onto conveyors and transported to a processing center
Longwall: Uses a rotating drum with “teeth,” which is pulled back and forth across a coal seam—the material then breaks loose and is transported to the surface
Room and pillar: Approximately half of the coal is left in place as pillars to support the roof of the active mining area.
Later, the pillars are removed and the mine collapses.
Environmental Damage from Mining
Acid mine drainage
Disruption of natural habitats
Chemicals from in situ leaching entering the water table
Disruption of soil microorganisms and, consequently, nutrient cycling processes
Dust released during the breakup of materials, causing lung problems and posing other health risks
Land subsidence
Large consumption and release of water
5.10: Impacts of Urbanization
Urbanization
Urbanization: It refers to the movement of people from rural areas to cities and the changes that accompany it.
Areas that are experiencing the greatest growth in urbanization are countries in Asia and Africa.
Pros Cons | |
Better educational delivery system. | Overcrowded schools. |
Better sanitation systems. | Sanitation systems have greater volumes of wastes to deal with. |
Large numbers of people generate high tax revenues. | Large numbers of poor people place strains on social services. |
Mass transit systems decrease reliance on fossil fuels—commuting distances are shorter. | Commuting times are longer because the infrastructure cannot keep with growth. |
Much of the pollution comes from point sources, enabling focused remediation techniques. | Since population densities are high, pollution levels are also high |
Recycling systems are more efficient. | Solid-waste buildup is more pronounced. Landfill space becomes scarce and costly. |
Urban areas attract industry due to the availability of raw materials, distribution networks, customers, and labor pool. | Higher population densities increase crime rates. Population increase may be higher than job growth. |
Urban Sprawl
Urban Sprawl: Also known as suburban, describes the expansion of human populations away from central urban areas into low-density and usually car-dependent communities.
Job sprawl: It has low-density, geographically spread-out employment patterns, with most jobs in a metropolitan area outside the central business district and increasingly in the suburbs.
Agricultural lands, which are/were frequently found immediately surrounding cities, are frequently taken from for urban sprawl.
Most housing is single-family homes on large lots with fewer stories than city homes, farther apart, and separated by lawns, landscaping, or roads.
Single-use development: Separate commercial, residential, institutional, and industrial areas. Thus, people live, work, shop, and play far apart and need a car.
Smart Growth
Smart growth: It promotes compact, transit-oriented, walkable, bicycle-friendly land use, neighborhood schools, and mixed-use development with a variety of housing options to slow urban sprawl and concentrate growth in a compact, walkable "urban villages."
It values long-range, regional considerations of sustainability.
Sustainable development strategies include the following:
Adopting mixed-use planning: Combining residential, commercial, cultural, institutional, and/or industrial uses in a specific location
Creating greenbelts and another undeveloped, wild, or agricultural land around cities
Providing property tax incentives to companies that locate in urban centers
Providing subsidies for mass transit systems and riders
Replacing abandoned buildings with green spaces reduces urban blight.
Urban or Planned Development?
Urban development: It is the process of designing and shaping the physical features of cities and towns with the goal of making urban areas more attractive, functional, and sustainable.
Some urban development strategies include the following:
Using recycled materials in waste-minimizing designs
Conserving energy through government and private industry rebates and tax incentives for solar and other clean energy
Improving indoor air quality
Locating buildings near multi-modal public transportation hubs like light rail, subways, and park and rides.
Preserving community history and culture while blending into its natural aesthetics
Using resource-efficient building techniques and materials
Conserving water through the use of xeriscaping
Urban Runoff
Urban runoff: It is surface runoff of rainwater created by urbanization.
This runoff is a major source of urban flooding and water pollution in urban communities worldwide.
Urban runoff results in the following:
Erosion causes runoff sedimentation, which settles to the bottom of water bodies and reservoirs, affecting water quality and storage capacity.
As urban heat transfers to streams and waterways, fish and wildlife suffer.
Runoff with gasoline, motor oil, heavy metals, trash, fertilizers, and pesticides.
Runoff containing gasoline, motor oil, heavy metals, trash, fertilizers, and pesticides
Constructing wetlands to naturally filter water before it enters lakes, rivers, and oceans.
Water retention-infiltration basins—shallow artificial ponds—infiltrate storm water into the groundwater aquifer through permeable soils.
Frequently using street-sweeping vacuums that can reduce the trash and other debris and pollutants that end up in runoff
Expanding urban parks and green spaces to increase natural infiltration
5.11: Ecological Footprints
Ecological Footprint: A measure of human demand on Earth’s ecosystems and is a standardized measure of demand for natural capital that may be contrasted with the planet’s ecological capacity to regenerate.
It represent the amount of biologically productive land and sea area that is necessary to supply the resources a human population consumes, and to assimilate associated waste.
5.12: Sustainability
Sustainability: It refers to the capacity for the biosphere and human civilization to coexist through the balance of resources within their environment.
To ensure that available resources are never depleted faster than those resources can be replaced.
IPAT formula
I = P × A × T
Sustainable agricultural practices, reducing consumption and waste, universal fishing quotas, and collaborative water management is needed to solve environmental issues caused by unsustainable resource use and pollution.
Threats to Sustainability
Earth-System Processes Control Variable Boundary Value Current Value Boundary Crossed Preindustrial Value | |||||
Biodiversity Loss | Extinction rate | 10 | >100 | yes | 0.1–1 |
Climate change | Atmospheric carbon dioxide concentration | 350 | 400 | yes | 280 |
Freshwater | Global human consumption of water | 4000 | 2600 | no | 415 |
Land use | % land surface converted to cropland | 15 | 11.7 | no | low |
Stratospheric ozone depletion | Dobson units | 276 | 283 | no | 290 |
Sustainable Agriculture
Sustainable agriculture: It emphasizes profitable, environmentally friendly, energy-efficient production and food systems that improve farmers' and the public's quality of life.
It prioritizes long-term solutions over short-term symptoms and land and rural community health.
Examples of sustainable agricultural practices include the following:
Developing ecologically-based pest management programs
Diversifying farms to reduce economic risks
Increasing energy efficiency in production and food distribution
Integrating crop and livestock production
Protecting the water quality
Reducing or eliminating tillage in a manner that is consistent with effective weed control
Rotating crops to enhance yields and facilitate pest management
Using cover crops, green manure, and animal manure to build soil quality and fertility
Using water and nutrients efficiently
Soil Conversion Techniques
Contour plowing: Plowing along the contours of the land in order to minimize soil erosion
No-till agriculture: Soil is left undisturbed by tillage and the residue is left on the soil surface.
Planting perennial crops: Perennials live for several years; e.g., fruit trees.
Strip cropping: Cultivation in which different crops are sown in alternate strips
Terracing: Make or form (sloping land) into a number of level flat areas resembling a series of steps
Windbreaks: Rows of trees that provide shelter or protection from the wind
Chapter 6: Energy Resources and Consumption
6.1: Introduction to Energy
Energy: Defined as the fundamental entity of nature that is transferred between parts of a system in the production of physical change within the system and is usually regarded as the capacity for doing work.
Sun: The source of energy for most of life on Earth.
It is heated to high temperatures by the conversion of nuclear energy to heat in its core by the process of nuclear fusion.
Human civilization requires energy to function. Humans obtain energy from resources such as fossil fuels, nuclear fuel, or renewable energy.
Forms of Energy
Chemical energy: It is stored in bonds between atoms in a molecule.
Electrical energy: It results from the motion of electrons.
Electromagnetic energy: This energy travels by waves.
Mechanical energy: Consists of potential and kinetic energies.
Potential Energy: Stored energy in any object.
Kinetic energy: Energy in motion.
Nuclear energy: It is stored in the nuclei of atoms, and it is released by either splitting or joining atoms.
Thermal Energy: the energy an object has because of the movement of its molecules.
Units of Energy/Power
British thermal unit (Btu): It is the amount of heat required to raise the temperature of 1 pound of water by 1°F.
Btu/hr: A ton in many air conditioning applications.
Horsepower (HP): Used in automobile industries.
1 HP = 746 watts
Kilowatt hour (kWh): A unit of power; a measure of energy used at a give moment.
A billing unit of energy delivered to consumers by electric utilities.
Law of Thermodynamics
First Law of Thermodynamics: The law of conservation of energy; energy can't be created nor destroyed.
Second Law of Thermodynamics: The total system work is always less than the heat supplied into the system.
Zeroth Law of Thermodynamics: If a body A is in thermal equilibrium with another body B, and body A is also in thermal equilibrium with a body C, then this implies that the bodies B and C are also in equilibrium with each other.
6.2: Renewable and Nonrenewable Resources
Renewable energy: Defined as energy that is collected from resources that are naturally replenished on a human time scale.
Renewable energy resources exist over wide geographical areas, in contrast to other energy sources that are concentrated in a limited number of countries.
Nonrenewable Energy Sources: Their use is not sustainable because their formation takes billions of years like fossil fuels.
Arguments used to defend the continued use of fossil fuels include the following:
Abundant supply, resulting in relatively low prices for consumers
Concentrated fuel with a high net-energy yield
Infrastructure already in place for extraction, processing, and delivery
Politics
Technology already exists for their use.
6.3: Fuel Types
Fossil Fuels: Fuels formed from past geological remains of living organisms.
Burning wood fuel: It creates the following by-products: carbon dioxide, heat, steam, water vapor, and wood ash.
Peat: It is an accumulation of partially decayed vegetation or organic matter, mostly wetland vegetation like mosses, sedges, and shrubs, that forms in acidic and anaerobic conditions.
Coal: Formed when dead plant matter that covered much of Earth’s tropical land surface at one time decays into peat and is then converted into coal by the heat and pressure of deep burial over millions of years.
Lignite: Often called brown coal, is the type most harmful to human health and is used almost exclusively as the primary fuel for electric power generation around the world.
Bituminous: Used primarily as fuel in steam-electric power generation.
Anthracite: Used primarily for residential and commercial space heating.
Clean Coal: Technology that attempts to mitigate emissions of carbon dioxide and other greenhouse gases that arise from the burning of coal for electrical power.
Carbon capture and storage (CCS): Pumps and stores CO2 emissions underground.
Natural gas: A fossil fuel formed when layers of buried plants and gases are exposed to intense heat and pressure over thousands of years.
Oil: A fossil fuel produced by the decomposition of deeply buried organic material (plants) under high temperatures and pressure for millions of years.
Cogeneration: Also known as combined heat and power (CHP), is an efficient technology to generate electricity and heat simultaneously at local facilities; otherwise, the heat produced from electricity generation is wasted.
Technologies used to remove pollutants from flue gases
Baghouse filters: Fabric filters that can be used to reduce particulates.
Burning pulverized coal at lower temperatures: Coal is crushed into a very fine powder and injected into a firebox.
Coal gasification: A process that turns coal and other carbon-based fuels into gas known as “syngas.”
Impurities are removed from the syngas before it is combusted, which results in lower emissions of sulfur dioxide, particulates, and mercury.
Cyclone separator: A method of removing particulates through rotational (spinning) effects and gravity.
Electrostatic precipitator: A filtration device that removes fine particles, like dust and smoke, from a flowing gas using an electrostatic charge.
Fluidized-bed combustion: A method of burning coal in which the amount of air required for combustion far exceeds that found in conventional burners.
This process can be used to reduce the amount of NOx, SOx, and particulates.
Scrubbers: Systems that inject chemical(s) into a dirty exhaust stream to “wash out” acidic gases.
It can also be used to reduce SOx and particulates from burning coal.
Sorbents: Activated charcoal, calcium compounds, or silicates can convert gaseous pollutants in smokestacks into compounds that baghouse filters, electrostatic precipitation, or scrubbers can collect.
6.4: Fossil Fuels
Law of Supply: All other factors being equal, as the price of a good or service increases, the quantity of goods or services that suppliers offer will increase, and vice versa.
As the price of an item goes up, suppliers will attempt to maximize their profits by increasing the quantity offered for sale.
Law of Demand: All other factors being equal, the quantity of the item purchased is inversely related to the price of the item.
Fossil fuels are formed over time from deposits of once-living organisms and take thousands of years to form.
Coal originally comes from land vegetation, which over millions of years decays and becomes compacted.
Natural gas was formed from the remains of marine organisms and is relatively abundant and clean when compared to coal and oil.
Oil is a liquid fossil fuel that formed from the remains of marine organisms, these deposits became trapped in small spaces in rock and sediment, which now can be accessed by drilling.
Other Fossil Fuel Nonrenewable Energy Resources
Methane Hydrates (Clathrates): These are recently discovered source of methane that form at low temperature and high pressure.
They are found:
On land in permafrost regions;
Beneath the ocean floor; and
On continental shelves.
Oil shale: An organic-rich, fine-grained sedimentary rock containing a solid mixture of organic chemical compounds (kerogen) from which liquid hydrocarbons (shale oil) can be produced.
Synfuels: Any fuel produced from coal, natural gas, or biomass through chemical conversion.
Tar sands: Contain bitumen—a semi-solid form of oil that does not flow. These are mined using strip mining techniques; in situ methods, using steam, can also be used to extract bitumen from tar sands.
Combustion
The combustion of any fossil fuel follows the following reaction:
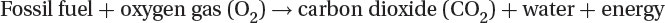
Carbon dioxide produced during fossil fuel combustion for heat and electricity generation is a major contributor to global CO2 emissions considered responsible for global warming due to its greenhouse gas effect.
Steps Involved from Fuels to Electricity
Extracting thermal energy from the fuel and using it to raise steam;
Converting the thermal energy of the steam into kinetic energy in the turbine; and
Using a rotary generator to convert the turbine’s mechanical energy into electrical energy.
Hydraulic Fracturing
Hydraulic fracturing: Also known as “fracking,” is an oil and gas well development process that typically involves injecting water, sand, and chemicals under high pressure into a bedrock formation via a well.
This process is intended to create new fractures in the rock as well as increase the size, extent, and connectivity of existing fractures.
It is commonly used in low-permeability rocks like sandstone, shale, and some coal beds to increase oil and/or gas flow to a well from petroleum-bearing rock formations.
6.5: Nuclear Power
Nuclear Fission
During nuclear fission, an atom splits into two or smaller nuclei along with by-product particles.
The reaction gives off heat.
If controlled, the heat that is produced is used to produce steam that turns generators that then produce electricity.
If the reaction is not controlled, a “meltdown” can result.
Nuclear Meltdown: A severe nuclear reactor accident that results in core damage from overheating.
Nuclear Fuels
U-235: Less than 1% of all-natural uranium on Earth.
Critical Mass: The minimum amount of U-235 required for a chain reaction.
U-238: The most common isotope of uranium and has a half-life of 4.5 billion years.
When hit by neutron, it eventually decays into Pu-239.
Pu-239: It has a half-life of 24,000 years and is produced in breeder reactors from U-238.
Its fission provides about one-third of the total energy produced in a typical commercial nuclear power plant.
Nuclear Components
Core: Contains up to 50,000 fuel rods.
Each fuel rod is stacked with many fuel pellets.
Fuel: Enriched (concentrated) U-235 is usually the fuel.
The fission of an atom of uranium produces 10 million times the energy produced by the combustion of an atom of carbon from coal.
Control rods: Move in and out of the core to absorb neutrons and slow down the reaction.
Moderator: It reduces the speed of fast neutrons, thereby allowing a sustainable chain reaction.
Coolant: Removes heat and produces steam to generate electricity.
6.6: Energy from Biomass
Biomass: It is biological material derived from living, or recently living, organisms that can be burned in large incinerators to create steam that is used for generating electricity.
It can be grown on marginal land that is not suitable for agriculture.
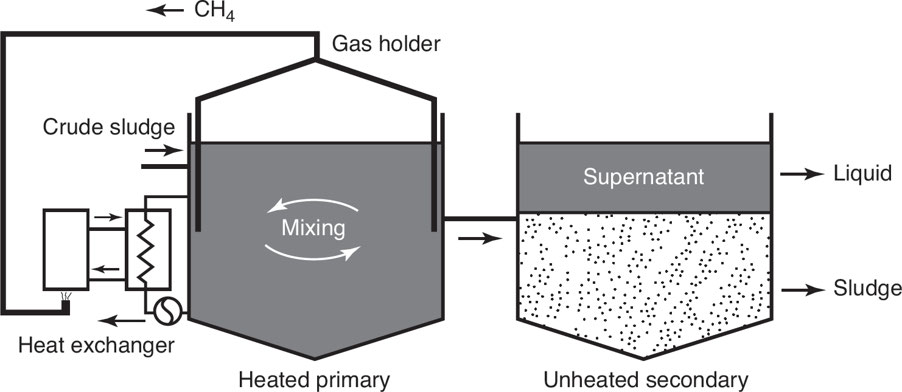
Anaerobic digestion: A collection of processes by which microorganisms break down biodegradable material, in the absence of oxygen, to produce methane gas, which is then burned to produce energy.
Reduces the reliance on coal and oil.
Reduces the impact of land disturbances required for coal mining.
Reduces the methane emissions from landfills that contribute to global warming.
Biofuel: A liquid fuel produced from living organisms.
These are biodegradable, can be converted into biodiesel or bioethanol to power vehicles.
It can be produced anywhere as opposed to fossil fuels.
It is a renewable energy source.
6.7: Solar Energy
Solar energy: It consists of collecting and harnessing radiant energy from the sun to provide heat and/or electricity.
Electrical power and heat is generated at home and at industrial sites through photovoltaic cells, solar collectors, or at a central solar-thermal plant.
Passive solar heating: It does not include any type of mechanical heating device and functions by incorporating building features that absorb heat and then release it slowly to maintain the temperature throughout the building.
Active solar heating: It generates more heat than passive systems, and relies on three components: a solar collector to absorb the solar energy, a solar storage system, and a heat transfer system.
Residential photovoltaic system: It consists of solar panels to absorb and convert sunlight into electricity, a solar inverter to change the electric current from DC to AC, and a battery storage and backup system.
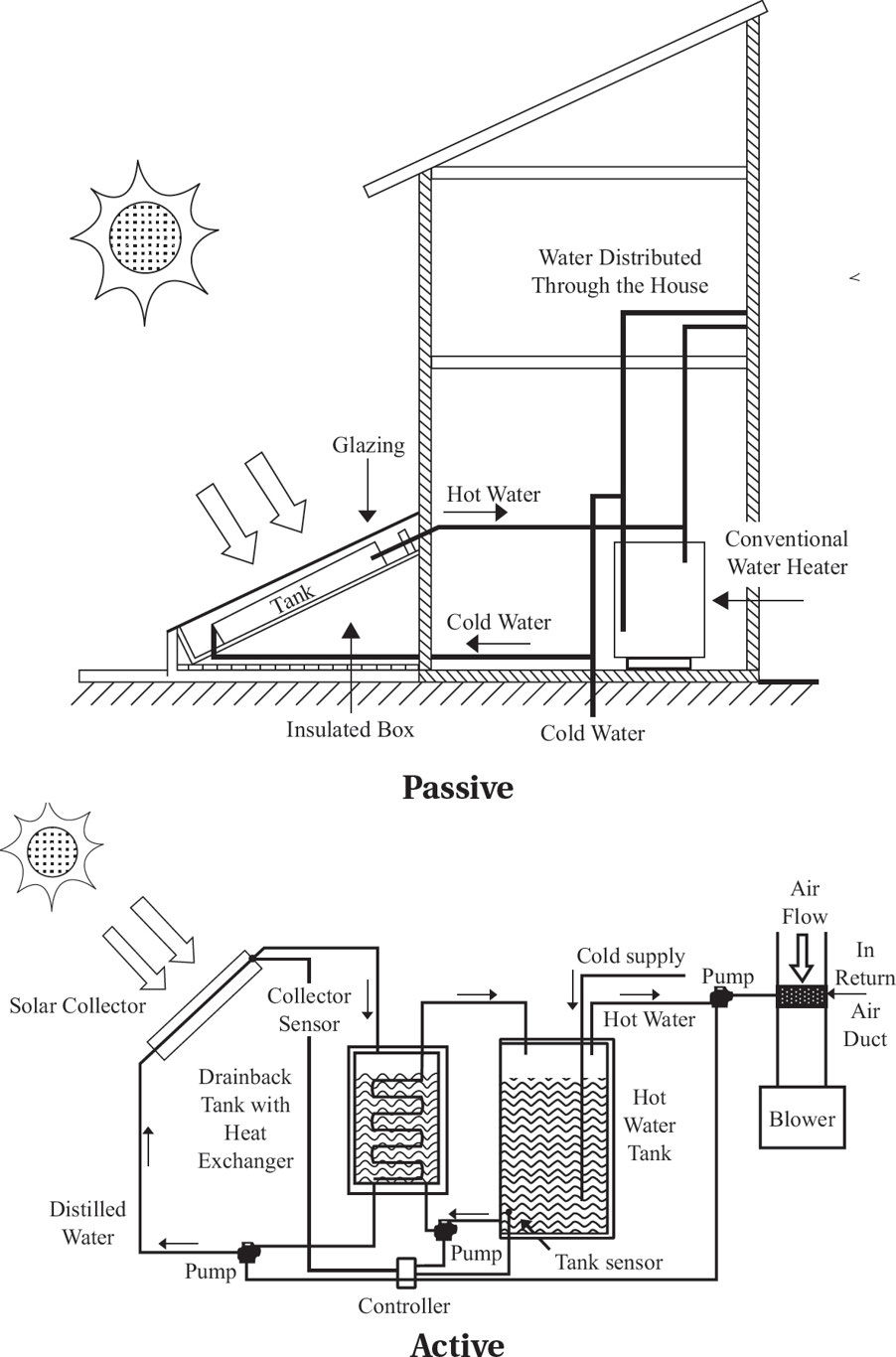
6.8: Hydroelectric Power
Dams: These are built to trap water, which is then released and channeled through turbines that generate electricity.
Hydroelectric generation accounts for approximately 44% of renewable electricity generation, and 6.5% of total electricity generation in the United States.
There are about 75,000 dams in the United States that block ~600,000 miles (~1 million km) of what had once been free-flowing rivers.
Advantages
Dams result in habitat destruction.
Dams help control flooding
Long life spans
Low operating and maintenance costs, which result in affordable electricity
Moderate to high net-useful energy
No polluting waste products
Provide water storage for municipal and agricultural use
Disadvantages
Dams are expensive to build.
Dams create large flooded areas behind the dam from which people are displaced.
Dams destroy wild rivers.
Dams destroy wildlife habitats and keep fish from migrating.
Dams reduce the amount of land available for agriculture.
Sedimentation behind the dam requires dredging.
Floods can be caused by the following:
Failures of dams, levees, and pumps
Fast snowmelt
Increased amounts of impervious surfaces, e.g., asphalt or concrete
Natural hazards, such as wildfires, reduce the supply of vegetation that absorbs rainfall
Prolonged heavy rainfall
Severe winds over water
Tsunamis
Unusually high tides and storm surges
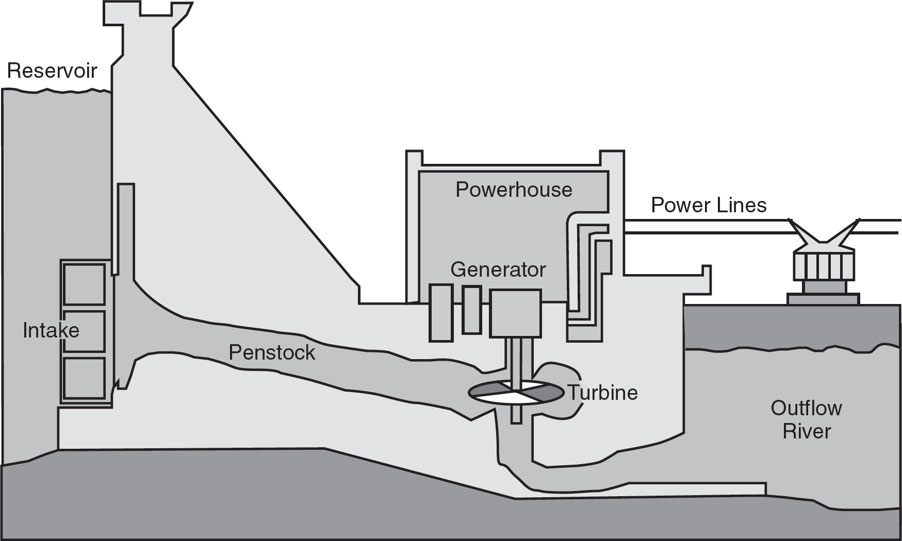
6.9: Geothermal Energy
Heat contained in underground rock and fluids from molten rock (magma), hot dry-rock zones, and warm-rock reservoirs produces pockets of underground steam and hot water that can be used to drive turbines, which can then generate electricity.
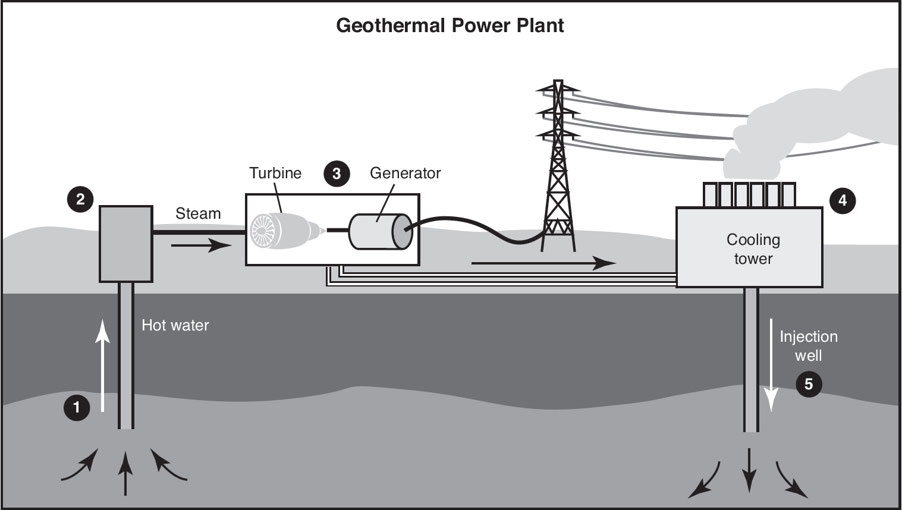
6.10: Hydrogen Fuel Cells
The hydrogen fuel cell operates similarly to a battery with two electrodes—oxygen passes over one and hydrogen passes over the other.
The hydrogen reacts with a catalyst to form negatively charged electrons and positively charged hydrogen ions (H+).
The electrons flow out of the cell to be used as electrical energy.
The hydrogen ions then move through a membrane, where they combine with oxygen and electrons to produce water.
Unlike batteries, fuel cells never run out.
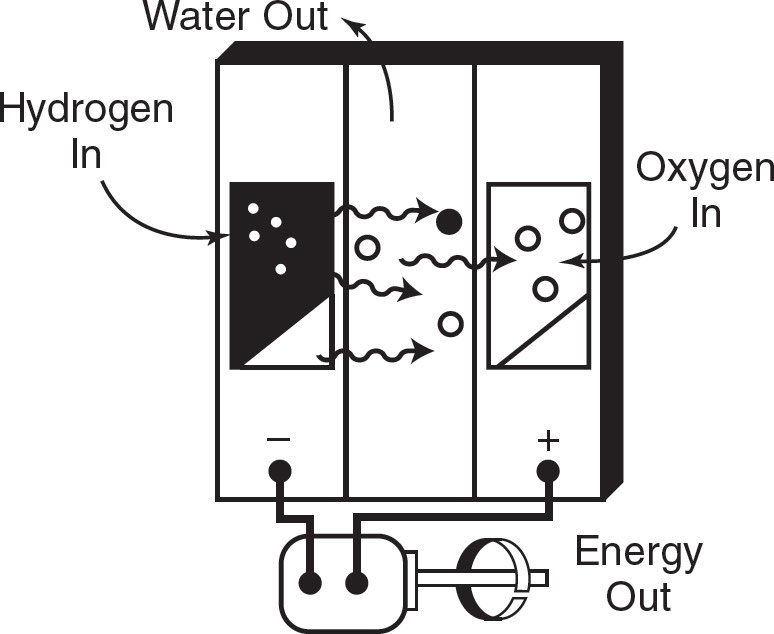
6.11: Wind Energy
Wind turbines work very simply: instead of using electricity to make wind—like a fan—wind turbines use wind to make electricity.
Wind turns the giant turbine blades, and then that motion powers generators.
Wind Farms: Wind turbines clustered together.
Using wind power is by far the most efficient method of producing electricity
One megawatt of wind energy can offset approximately 2,600 tons of CO2.
About 6% of the electrical demand in the United States is now produced from wind energy.
The current capacity of wind power in the United States powers approximately 20 million homes.
Offshore wind represents a major opportunity to provide power to highly populated coastal cities.
The largest turbines can harness energy to power 600 American homes.
The country with the largest wind energy installed capacity is China, followed by the United States.
There has been a 25% increase in wind turbine use in the last decade, but wind energy only provides a small percentage of the world’s energy.
6.12: Energy Conservation
Add extra insulation and seal air leaks.
Improving attic insulation and sealing air leaks can save 10% or more on annual energy bills.
Change to a programmable HVAC thermostat.
A programmable thermostat can save as much as 15% on heating and cooling costs.
Change to more efficient LED lighting.
LED lights do not contain mercury and can be disposed of with the regular household trash.
Minimize phantom loads.
Phantom Load: Refers to the energy that an appliance or an electronic device consumes when it is not actually turned on.
75% of the electricity used to power home electronics is consumed while the products are turned off.
Use more energy-efficient appliances.
Chapter 7: Atmospheric Pollution
7.1: Introduction to Air Pollution
Air pollution: It occurs when harmful or excessive quantities of substances are introduced into Earth’s atmosphere.
Parts per million (ppm): The most common form of expressing air pollutants.
Primary Pollutants: Emitted directly into the air.
Secondary Pollutants: Result from primary air pollutants’ reacting together and forming new pollutants.
Point source air pollution: It occurs when the contaminant comes from an obvious source.
Non-point source air pollution: It occurs when the contaminant comes from a source that is not easily identifiable or from a number of sources spread over a large, widespread area.
Criteria air pollutants: These are a set of eight air pollutants that cause smog, acid rain, and other health hazards and are typically emitted from many sources in the industry, mining, transportation, power generation, and agriculture.
7.2: Atmospheric CO2 and Particulates
Industrial smog: Trends to be sulfur-based and is also called gray smog.
Formation of Industrial Smog
Carbon in coal or oil is burned in oxygen gas to produce carbon dioxide and carbon monoxide gas.
Unburned carbon ends up as soot or particulate matter (PM).
Sulfur in oil and coal reacts with oxygen gas to produce sulfur dioxide.
Sulfur dioxide reacts with oxygen gas to produce sulfur trioxide.
Sulfur trioxide reacts with water vapor in the air to form sulfuric acid.
Sulfuric acid reacts with atmospheric ammonia to form brown, solid ammonium sulfate.
Carbon Monoxide (CO)
Carbon monoxide: It is a colorless, odorless, and tasteless gas that is slightly less dense than air and is produced from the partial oxidation of carbon-containing compounds.
It forms when there is not enough oxygen to produce carbon dioxide.
Carbon monoxide is present in small amounts in the atmosphere, primarily as a product of the following:
Natural and man-made fires.
Photochemical reactions in the troposphere.
The burning of fossil fuels
Volcanic activity
Methods to reduce carbon monoxide pollution include the following:
Building more public transportation infrastructure
Requiring catalytic converters on all cars worldwide; however, this only converts carbon monoxide to carbon dioxide—a greenhouse gas
Switching to renewable energy sources
Lead (Pb)
Lead: It is used in building construction, lead-acid batteries for vehicles, bullets and shot fishing weights, solder, and shields for radiation.
Exposure to lead can occur from inhalation of polluted air and dust and from the ingestion of lead in food and/or water.
Symptoms of lead poisoning include failure of the blood to make hemoglobin, which results in anemia disruptors, mental retardation and disabilities, hypertension, miscarriages and/or premature births, and even death at relatively low concentrations.
Nitrogen Oxides
Nitrogen Oxide: A generic term for nitric oxide and nitrogen dioxide, which are produced from the reaction of nitrogen and oxygen gases in the air.
These gases are formed whenever nitrogen occurs in the presence of high-temperature combustion.
Nitrous oxide: It is a major air pollutant, with levels of N2O having increased by more than 15% since 1750.
It causes ozone depletion.
It is formed by denitrification and nitrification.
Ozone
Ozone: It is an inorganic molecule with the chemical formula O3, and tropospheric (ground-level) ozone is a secondary air pollutant.
Tropospheric ozone: It does not have strong global effects, but instead is more influential in its effects on smaller, more localized areas.
Tropospheric ozone can have the following effects:
Cause asthma and bronchitis
Harm lung function and irritate the respiratory system
Result in heart attacks and other cardiopulmonary problems
Suppress the immune system.
Peroxyacyl Nitrates (PANs)
Peroxyacyl Nitrates (PANs): These are secondary pollutants. Because they break apart quite slowly in the atmosphere into radicals nd NO2, PANs are able to move far away from their urban and industrial origin.
It causes:
Eye irritation
Impaired immune systems
Inhibited photosynthesis
Reduced crop yields by damaging plant tissues
Respiratory problems
Methods to reduce PANs include the following:
Limiting wood-burning fireplaces and stoves in new home construction
Reducing smokestack emissions through baghouse filters, cyclone precipitators, scrubbers, and/or electrostatic precipitators
Reducing the incineration of municipal and industrial wastes
Reducing the reliance on fossil fuels, especially oil and coal
Sulfur Dioxides
Sulfuric Dioxide: A colorless gas with a penetrating, choking odor that readily dissolves in water to form an acidic solution.
Sulfur dioxide emissions come from power stations, oil refineries, and large industrial plants burning fossil fuels.
It is toxic to a variety of plants and reduces crop yields.
Sulfur dioxide, emitted in sufficient quantities at low or ground level, can combine with air moisture to form an acid solution that dissolves stonework.
It irritates the throat and lungs, and, if there are fine dust particles in the air, can damage the respiratory system.
Steps that can be taken to reduce the amount of SO2 in the atmosphere include the following:
Fluidized gas combustion
Using only low-sulfur coal
Using scrubbers in the smokestacks
Washing the coal
Suspended Particulate Matter
Suspended particulate matter (PMx): It is microscopic solid or liquid matter suspended in Earth’s atmosphere.
The “x” refers to the size of the particle.
The smaller and lighter a particle is, the longer it will stay in the air.
Larger particles tend to settle to the ground by gravity in a matter of hours, whereas the smallest particles can stay in the atmosphere for weeks and are mostly removed by precipitation.
Particulate Matter
affects the diversity of ecosystems;
changes the nutrient balance in coastal waters and large river basins;
depletes the nutrients in the soil;
damages sensitive forests and farm crops;
increases health issues with humans and animals
makes lakes and streams more acidic.
Airborne particulate matter can be reduced by:
conserving energy to reduce demands on power plants;
increasing air-quality standards for emissions of particulate matter from smokestacks;
increasing automobile emission standards;
limiting the use of household and personal products that cause fumes;
not burning leaves and other yard waste;
not using wood in fireplaces
Naturally Occurring PMx Anthropogenic Occurring PMx | |
Dust storms | Burning of fossil fuels—power plants |
Forest and grassland fires | Incineration of wastes |
Sea spray | Soil erosion—desertification, deforestation |
Volcanoes | Vehicle exhaust |
Volcanic Organic Compounds
Volcanic Organic Compounds (VOCs): These are organic chemicals that have a high vapor pressure (easily evaporate) at ordinary room temperature.
Their high vapor pressure results from a low boiling point, which causes large numbers of molecules to evaporate and enter the surrounding air.
Health effects of “sick building” syndrome include
cancer;
damage to the liver, kidney, and central nervous system;
eye, nose, and throat irritation; and
headaches, loss of coordination, and nausea.
7.3: Photochemical Smog
Photochemical smog: It is catalyzed by ultraviolet (UV) radiation, tends to be nitrogen-based, and is referred to as brown smog.
Forming Photochemical Smog
6 A.M.–9 A.M.: As people drive to work, concentrations of nitrogen oxides and VOCs increase.
9 A.M.–11 A.M.: As traffic begins to decrease, nitrogen oxides and VOCs begin to react, forming nitrogen dioxide (NO2).
11 P.M.–4 P.M.: As the sunlight becomes more intense, nitrogen dioxide is broken down and the concentration of ozone (O3) increases.
Nitrogen dioxide also reacts with water vapor to produce nitric acid (HNO3) and nitric oxide (NO).
Nitrogen dioxide can also react with VOCs released by vehicles, refineries, and gas stations to produce toxic PANs (peroxyacyl nitrates).
4 P.M.–Sunset: As the sun goes down, the production of ozone is halted.
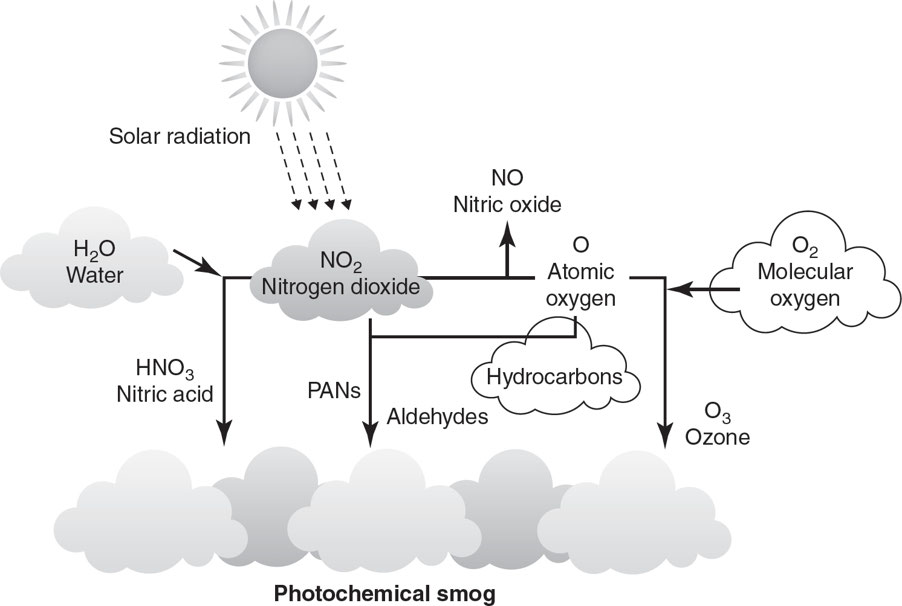
7.4: Thermal Inversion
Thermal inversions: These occur when air temperature rises with height instead of falling.
This effect traps pollution like smog close to the ground, which may harm human health.
This usually happens at night when solar heating stops and the surface cools, cooling the atmosphere above it.
A warm air mass moving over a colder one traps the cooler air below and stills the air, trapping dust and pollutants and increasing their concentrations.
Antarctica has a nearly constant temperature inversion.
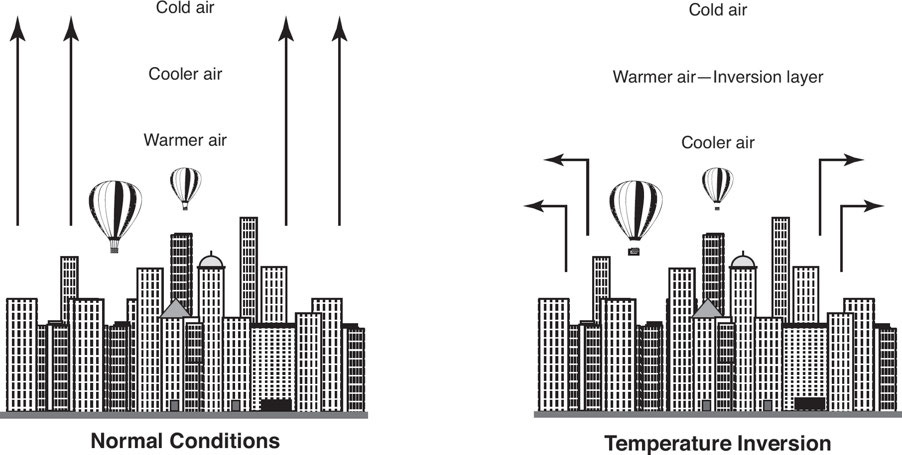
7.5: Outdoor and Indoor Air Pollutants
“Sick building” syndrome (SBS): It is a term used to describe a combination of ailments associated with an individual’s place of work or residence.
Asbestos: It is inexpensive, durable, and flexible and naturally acts as an insulating and fireproofing agent.
Carbon monoxide poisoning: It is the most common type of fatal indoor air poisoning in many countries because it easily combines with hemoglobin to block the blood’s oxygen-carrying capacity.
Formaldehyde: It is an organic chemical that is prevalent in the indoor environment and is a carcinogen that is linked to lung cancer.
Radon: It is an invisible radioactive gas that results from the radioactive decay of radium, which can be found in rock formations beneath buildings.
Cigarette smoke: It contains almost 5,000 chemical compounds, including 60 known carcinogens (cancer-causing chemicals), one of which is dioxin.
Remediation Steps to Reduce Indoor Air Pollutants
Add plants that absorb toxins.
Do not allow smoking indoors.
Install air purification systems and ensure adequate fresh air ventilation when temperatures permit.
Maintain all filters and vents.
Monitor humidity levels to reduce mold and mildew.
Test for radon gas and other dangerous indoor pollutants.
Use “green” cleaning products.
Use natural pest-control techniques.
7.6: Reduction of Air Pollutants
Controlling Air Pollution/Pollution-Control Devices
Catalytic converter: It is an exhaust emission control device that converts toxic chemicals in the exhaust of an internal-combustion engine into less harmful substances.
Catalyst: It stimulates a chemical reaction in which by-products of combustion are converted to less toxic substances by way of catalyzed chemical reactions.
Most present-day vehicles that run on gasoline are fitted with a “three way” converter, since it converts the three main pollutants:
Oxidation of carbon monoxide to carbon dioxide:
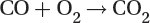
Oxidation of unburned hydrocarbons to carbon dioxide and water:
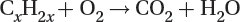
Reduction of nitrogen oxides to nitrogen and oxygen:
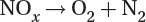
Catalytic converters remove hydrocarbons and other harmful emissions, but they do not reduce fossil fuel-produced carbon dioxide.
Remediation Steps to Reduce Air Pollution
Ban open burning of waste.
Buy smaller cars and energy-efficient appliances.
Decrease unnecessary travel.
Distribute solar cook stoves to developing countries to replace wood and coal.
Drive within the speed limit and keep tires inflated.
Institute flexible work shifts.
Maintain vehicle properly with regular tune-ups and oil changes.
Reduce idling and turn off engines while waiting.
Use mass transit systems or carpool when possible.
Toughen Corporate Average Fuel Economy (CAFE) standards.
Toughen legislation to reduce sulfur content in fuel.
Use fans instead of air conditioners.
Use fluorescent or LED lighting.
When buying a car, consider its fuel efficiency.
7.7: Acid Rain (Deposition)
Acid deposition: It occurs when atmospheric chemical processes transform sulfur and nitrogen compounds and other substances into wet or dry deposits on Earth.
Dry Deposition: In dry areas, acidic chemicals in the air may become dust or smoke and stick to the ground, buildings, homes, cars, and trees, which rainstorms wash away, increasing acidic runoff.
Wet Deposition: Acid rain, fog, and snow. As this acidic water flows over and through the ground, it affects a variety of plants and animals.
Acid rain: It causes acidification of lakes and streams.
It damages trees at high elevations and many sensitive forest soils by nitrogen saturation and acidification that harms decomposers and mycorrhizal fungi.
Acid shock: Caused by rapid melting of snow pack with dry acidic particles, raises lake and stream acid concentrations five to ten times higher than acidic rainfall.
Acid deposition due to sulfur dioxide begins with sulfur dioxide being introduced into the atmosphere by burning coal and oil, smelting metals, organic decay, and ocean spray.
It then combines with water vapor to form sulfurous acid which then reacts with oxygen to form sulfuric acid.
Acid deposition due to nitrogen oxides begins with nitrogen oxides formed by burning oil, coal, or natural gas.
They are also found in volcanic vent gases and are formed by forest fires, bacterial action in the soil, and lightning-induced atmospheric reactions.
Effects of Acid Deposition
Acid shock
An increase in fish kills.
Changes in animal life due to changes in vegetation
Vegetation changes due to soil pH and ecosystem changes affect food webs.
Increased leaching of soil nutrients
Increased solubility of toxic metals, including methyl mercury, lead, and cadmium
Reduced buffering capacity of the soil
Heat Islands and Air Pollution
Urban heat islands: These occur in metropolitan areas that are significantly warmer than their surroundings.
Since warmer air can hold more water vapor, rainfall can be as much as 30% greater downwind of cities when compared with areas upwind.
Reasons for higher urban temperatures are as follows:
Air conditioning, transportation, lighting, and other fuels generate heat.
Urban impervious materials reduce the cooling effect of soil and leaf evaporation and tree shading.
Buildings block Earth's thermal radiation.
There is a lack of vegetation and standing water.
More black asphalt and building surfaces absorb heat and reduce sunlight reflectivity.
Street Canyon: A place where the street is flanked by buildings on both sides, creating a canyon-like environment.
High levels of pollution in urban areas can also create a localized greenhouse effect.
Urban heat islands can directly influence the health and welfare of urban residents who cannot afford air conditioning
7.8: Noise Pollution
Noise pollution: It is an unwanted human-created sound that disrupts the environment.
The dominant form of noise pollution is from transportation sources.
Effects of Noise Pollution
Sensory hearing loss is caused by damage to the inner ear and is the most common form associated with noise pollution.
Excessive noise can cause:
a decrease in alertness and the ability to memorize;
anxiety and nervousness;
cardiovascular problems, which manifest as an accelerated heartbeat and high blood pressure; and
gastrointestinal problems.
Techniques to Reduce Roadway Noise
Create computer-controlled traffic flow devices that reduce braking and acceleration, and implement changes in tire designs.
Create noise barriers.
Introduce newer roadway surface technologies.
Limit times for heavy-duty vehicles.
Place limitations on vehicle speeds.
Techniques to Reduce Aircraft Noise
Develop quieter jet engines.
Reschedule takeoff and landing times.
Techniques to Reduce Industrial Noise
Create new technologies in industrial equipment.
Install noise barriers in the workplace.
Control residential noise, such as power tools, garden equipment, and loud entertainment equipment, through local laws and enforcement.
Chapter 8: Aquatic and Terrestrial Pollution
8.1: Sources of Water Pollution
Water pollution: It is the contamination of water bodies.
This form of environmental degradation occurs when pollutants are directly or indirectly discharged into water bodies without adequate treatment to remove harmful compounds.
Sources of Water Pollution
Point source pollution: Release pollutants from known locations, such as discharge pipes, that are regulated by federal and state agencies.
Non-point source pollution: A combination of pollutants from a large area rather than from specific identifiable sources
Thermal Pollution: The degradation of water quality by any process that changes ambient water temperature.
8.2: Human Impacts on Ecosystem
Cultural eutrophication: It is the process whereby human activity increases the amount of nutrients entering surface waters.
Ecological Effects of Cultural Eutrophication
Changes in species composition and dominance
Decomposed algae by anaerobic bacteria release toxic gases
Decreased biodiversity in water bodies
Dissolved oxygen (DO) depletion (hypoxia), resulting in fish kills
Increase in algal blooms
Increase in turbidity
Increased phytoplankton biomass
Toxic phytoplankton species
Human Activities That Contribute to Cultural Eutrophication
Discharge from water treatment facilities that do not have the capacity to handle nutrient and biodegradable waste discharge
Fertilizers and pesticides from residential and agricultural runoff
Sewer and drainage overflows can occur when the rainfall amount exceeds the wastewater treatment capacity
Use of household products that contain phosphates
Steps for Controlling Cultural Eutrophication
Controlling runoff from feedlots
Controlling the application and timing of applying fertilizer
Constructing wastewater lagoons and retention ponds near agricultural areas
Planting vegetation (buffer zones) along streambeds, which slows erosion and absorbs some of the excess nutrients
Updating building codes to utilize permeable pavement to absorb the excess urban runoff
Upgrading existing water treatment plants to better control nitrate and phosphate pollution through tertiary standards and other advanced technologies
Using monetary and tax incentives to convert existing watering systems to drip irrigation and to replace landscaping with native vegetation that is less water-demanding
Biodegradable Wastes
Nitrates: These are water-soluble and are found in fertilizers, which can remain on fields and accumulate, leach into groundwater, or end up in surface runoff and cause algal blooms in surface waters, resulting in decreased dissolved oxygen levels.
Phosphates: These are also a component of fertilizers; however, they are not water-soluble, and they adhere to soil particles.
Disease-causing microorganisms: Such as bacteria, viruses, and protozoa, can result in swimmers getting sick and shellfish becoming contaminated.
Mining
Cyanide is intentionally poured onto piles of mined rock to chemically extract gold.
Mining companies in developing nations dump waste into rivers and other waterways.
Mining releases earth-locked heavy metals and sulfur compounds.
Rainwater on tailings pollutes freshwater.
Effects of Oil Spills
Seabirds ingest their feather oil while preening, damaging their kidneys and livers.
Due to their limited foraging, they dehydrate quickly.
Oil floats on water, blocking sunlight from reaching marine plants and phytoplankton, affecting the ecosystem's food web.
Oil penetrates seabird feathers, making them less buoyant and more susceptible to temperature changes.
Dispersants, sorbents, and detergents disperse, absorb, or clump oil into sinking gel-like agglomerations.
Controlled burning, booming, skimming, and/or vacuuming oil from the surface or shoreline.
The use of microorganisms to break down the oil.
Great Pacific Garbage Patch
Great Pacific Garbage Patch: A large system of rotating ocean currents of marine litter in the central North Pacific Ocean and is characterized by high concentrations of floating plastics, chemical sludge, and other debris that have been trapped by the currents of the North Pacific Gyre.
Great Patch: Formed as a result of marine pollution gathered by oceanic currents as the gyre’s rotational pattern drew in waste material from across the North Pacific Ocean.
As materials are captured in the currents, the wind-driven surface currents gradually move floating debris toward and trap it in the center of the gyre.
As the plastic photodegrades into smaller and smaller pieces, it remains as plastic polymers leaching toxic chemicals into the upper water column.
As the plastic further disintegrates, it becomes small enough to be ingested by aquatic organisms and birds near the ocean’s surface and eventually enters the marine food chain.
8.3: Persistent Organic Pollutants (POPs)
Persistent Organic Pollutants: These are organic (carbon) compounds that are resistant to environmental degradation through chemical or biological processes or decomposition due to light.
It has been observed to persist in the environment, be capable of long-range transport, bioaccumulate in human and animal tissue, biomagnify in food chains, and have potentially significant impacts on human health and the environment.
Chemical characteristics of POPs include the following:
Ability to travel long distances through the atmosphere before being deposited on Earth.
A tendency to evaporate easily in hot regions and accumulate in cold regions, where they tend to condense and persist
High molecular masses
High-fat solubility, which makes them able to pass through biological membranes and bioaccumulate in the fatty tissues of living organisms
Low water solubility.
Urban Runoffs are the major source of urban flooding and water pollution in urban communities worldwide. It also creates:
create microclimates due to the high heat capacity of asphalt
fragment habitats;
increase groundwater depletion as water does not infiltrate into the soil to recharge aquifers; and
reduce biodiversity and seriously impact food webs in the area since there is less vegetation available for primary consumers.
Water Quality—Water Testing
Water quality: Refers to the chemical, physical, and biological characteristics of water and is a measure of the condition of the water relative to the requirements of one or more biotic species and/or to any human need or purpose.
Water testing: It is a broad description for various procedures that are used to analyze water quality.
Water Quality Tests
Alkalinity: It measures the sum of the bicarbonate, carbonate, and hydroxide ions in the water, which raise the pH.
The water source's ability to resist pH changes can increase fish egg, larva, and fry survival rates.
Ammonia: When found in natural water, is regarded as an indicator of pollution.
It is rapidly oxidized by certain bacteria in natural water systems into nitrite and nitrate.
Biological Oxygen Demand (BOD): It gives an approximation of the level of biodegradable waste in water.
Carbon Dioxide: Aquatic vegetation, ranging from phytoplankton to large rooted plants, depends upon carbon dioxide and bicarbonates in the water for growth.
When the oxygen concentration falls the carbon dioxide concentration increases and the pH increases.
High CO2 levels also make it difficult for fish to use the limited amount of oxygen present in the water and to discharge the CO2 in their bloodstream.
Low CO2 levels also result in a decreased rate of photosynthesis.
Dissolved Oxygen (DO): If its level is too low, it indicates possible water pollution and shows a potential for further pollution downstream because the ability of the stream to self-cleanse will be reduced.
Coliforms: These are a form of bacteria that are found in the intestines of warm-blooded animals; their presence in lakes, streams, and rivers is a sign of untreated sewage in the water.
Fecal coliforms: They can get into the water from untreated human sewage or from farms and runoff from animal feedlots.
Heavy Metals (Pb, Hg, Cd, Se)
As water becomes more acidic, metal solubility increases, and the metal particles become more mobile.
Metals can become “locked up” in bottom sediments, where they remain for many years.
Heavy metals are non-biodegradable and can cause decreased reproductive rates and birth defects.
Nitrate: This gets reduced to nitrites, which can be harmful to humans and fish.
Nitrite: Occurs in water as an intermediate product in the biological breakdown of organic nitrogen being produced either through the oxidation of ammonia or the reduction of nitrate.
pH
Changes in water pH can result in increased mortality of eggs and juveniles, decalcification of bone, and physiological stress.
Pollution tends to make water acidic and increases the solubility of heavy metals.
Most bodies of water have the highest biological diversity when the pH is near 7, with natural waters having pH values from 5.0 to 8.5.
Fresh rainwater may have a pH of 5.5 to 6.0 due to carbon dioxide dissolving in the water, making a weak carbonic acid solution.
Phosphates: These are essential nutrients for aquatic plants, but only in very low concentrations.
Excessive amounts of phosphorus build up easily, and small amounts can contaminate large volumes of water.
Salinity
Proper salinity levels are required to maintain osmotic pressure for living cells.
Decreased salinity also results in decreased DO and decreased viability of eggs and larvae.
Solids
A steady concentration of dissolved minerals is necessary to maintain the osmotic balance within the cells of organisms.
High levels of total dissolved solids (TDS) can affect water clarity and photosynthesis and lead to a decline in the quality and taste of drinking water.
Temperature
Higher water temperatures lower the amount of dissolved oxygen:
gases are less soluble in warmer water; and
warmer water increases the metabolic rate of aquatic organisms.
Higher temperatures also increase an organism’s sensitivity to toxic wastes and diseases.
Total hardness: It measures the total concentration of calcium and magnesium ions in the water.
Increased concentrations of these ions can increase the solubility of heavy metal ions in water and affect the water’s buffering capacity.
Turbidity: It is a measure of how light is scattered in the water column due to solids that do not dissolve but are small enough to be suspended in the water.
Drinking Water Treatment Methods
Absorption: When one substance enters completely into another.
Adsorption: When one substance just hangs onto the outside of another.
Disinfection: Using chemicals and/or cleansing techniques that destroy or prevent the growth of organisms that are capable of infection.
Filtration: Removes clays, natural organic matter, precipitants, and silts from the treatment process.
It clarifies water and enhances the effectiveness of disinfection.
Flocculation sedimentation: A process that combines small particles into larger particles that then settle out of the water as sediment
Ion exchange: Removes inorganic constituents and can be used to remove arsenic, chromium, excess fluoride, nitrates, radium, and uranium
8.4: Endocrine Disruptors
Gland: An organ that secretes particular chemical substances for use in the body or for discharge into the surroundings.
Endocrine System: A network of glands that make the hormones that help cells communicate with each other and is responsible for almost every cell, organ, and function in both humans and animals.
Endocrine disruptors: These are chemicals that can interfere with endocrine or hormonal systems and can cause behavior, learning and developmental disorders, birth defects, cancerous tumors, and loss of fertility.
Bisphenol A (BPA): Used in plastic manufacturing and epoxy.
Dioxins: By-product of herbicide production and paper bleaching, and released during burning wastes and wildfires.
Phthalates: Used to make plastics more flexible.
Polychlorinated biphenyls (PCBs): Used to make electrical equipment, heat transfer fluids and lubricants.
8.5: Human Impacts on Wetlands and Mangroves
Wetland: It is a place where the land is covered by water, which can be freshwater, saltwater, or brackish water.
It includes marshes, ponds, the edge of a lake or ocean, the delta at the mouth of a river, and low-lying areas that frequently flood.
Wetlands support high animal concentrations and serve as breeding grounds and nurseries for many species, making their destruction a major environmental issue.
Wetlands also allow for the cultivation of rice, a food source for half of the world’s population.
Mangrove: A shrub or small tree that grows in slightly salty (brackish) water formed by seawater mixing with freshwater in estuaries.
They have a complex salt filtration system and root system to survive salt water, low-oxygen mud, and wave action.
Since the Industrial Revolution, humans have had an increasingly negative impact on one of the most productive ecosystems on Earth through:
Diking and dredging;
Filling in these sensitive areas for urban development impacts water quality and runoff; and
An increase in hurricanes resulted in more frequent sea surges.
8.6: Bioaccumulation and Biomagnification
Bioaccumulation: It is the increase in the concentration of a pollutant within an organism.
The rate at which a given substance bioaccumulates depends upon the following:
The mode of uptake.
The degree of fat solubility of the pollutant
The rate at which the substance is eliminated from the organism
The transformation of the substance by metabolic processes
The lipid (fat) content of the organism
Biomagnification: It is the increasing concentration of a substance in the tissues of organisms at successively higher trophic levels within a food chain.
For biomagnification to occur, the following must be true:
The pollutant must be long-lived.
The pollutant must be mobile.
The pollutant must be soluble in fats.
The pollutant must be biologically active.
8.7: Solid Waste Disposal
Municipal solid waste (MSW): More commonly known as trash or garbage—consists of everyday items that are used and then thrown away.
Hazardous Wastes: Paints, chemicals, pesticides, etc.
These wastes can take hundreds of years to decompose.
Organic Wastes: Kitchen wastes, vegetables, flowers, leaves, or fruits.
Usually decompose within two weeks.
Wood can take 10 to 15 years to decompose.
Radioactive Wastes: Spent fuel rods and smoke detectors.
These can take hundreds of thousands of years to decompose.
Recyclable Wastes: Glass, metals, paper, and some plastics.
Paper decomposes in 10 to 30 days, while glass does not decompose.
Metals decompose in 100 to 500 years.
Some plastics can take up to 1 million years to decompose.
Soiled Wastes: Hospital wastes.
Cotton and cloth can take two to five months to decompose.
Anaerobic Digestion
Microorganisms: Are used to break down biodegradable material and sewage sludge in the absence of oxygen.
It is a renewable energy source.
It reduces the amount of organic matter, which might otherwise be destined to be dumped at sea or in landfills or burned in incinerators.
It reduces or eliminates the energy footprint of waste treatment plants.
It reduces the methane emission from landfills.
It is best suited for organic material and is commonly used for industrial effluent, wastewater, and sewage sludge treatment.
Nutrient-rich digestate can be used as fertilizer.
Waste Solutions and Energy Recovery
Incineration: A waste treatment process that involves the combustion of substances contained in waste materials and the conversion of the waste into ash, flue gas, and heat.
Global waste trade: It is the international trade of waste between countries for further treatment, disposal, and/or recycling.
Toxic or hazardous wastes are often exported from developed countries to developing countries.
Ocean dumping: The deliberate disposal of municipal and/or hazardous wastes at sea.
Sanitary landfills: Method of waste disposal where the waste is buried either underground or in large piles, and where waste is isolated from the environment until it is safe.
Reducing: Lessening the number of hazardous wastes by substituting and using products that are more “Earth-friendly.”
Freon®: Its molecular structure contains chlorine, which seriously degrades the stratospheric ozone layer.
Puron®: Substitutes fluorine for chlorine, and has less of an impact on the stratospheric ozone layer.
Hazardous Wastes
Radioactive wastes: Usually a by-product of nuclear power generation and other applications of nuclear fission, such as research and medicine.
This waste is hazardous to most forms of life and the environment and is regulated by government agencies to protect human health and the environment.
Low-level radioactive wastes: Contain low levels of radiation and remain dangerous for a relatively short time.
High-level radioactive wastes: Contain high levels of radiation and remain dangerous for a very long time
Reactive wastes: These are wastes that are unstable under normal conditions. Reactive wastes can cause explosions, gases, toxic fumes, or vapors when heated, compressed, or mixed with water.
Source-specific wastes: These are wastes from specific industries.
Teratogens: These are substances found in the environment that can cause birth defects.
Toxic wastes: These are wastes that are harmful or fatal when ingested or absorbed.
When disposed, these toxins may leach and pollute the groundwater.
Handling Hazardous Wastes
Landfill capping: A containment technology that forms a barrier between the contaminated media and the surface, protecting humans and the environment from its harmful effects and limiting its migration.
Hazardous waste caps consist of three layers:
An upper topsoil layer
A compacted soil barrier layer
A low-permeability layer made of a synthetic material
Hazardous waste landfills: Excavated or engineered sites for the final disposal of non-liquid hazardous waste are selected and designed to minimize environmental release.
Permanent storage: Isolates hazardous waste from the environment by condensing or concentrating it.
Methods Used to Isolate and Store Hazardous Wastes
Salt dome and bed formations, underground caves, and mines are geologic repositories.
Surface impoundments: These are natural topographic depressions, man-made excavations, or diked areas that are used for temporary storage and/or for the treatment of liquid hazardous waste.
Injection wells: These stores fluid deep underground in geologically stable, porous rock formations, such as sandstone or limestone, or into or below the shallow soil layer.
Waste piles: These are non-containerized piles of solid, non-liquid hazardous waste that are used for temporary storage or treatment.
Reduction and cleanup of hazardous wastes: These can occur by producing less waste, converting the hazardous material to less hazardous or nonhazardous substances, and placing the toxic material into perpetual storage.
Brownfield: It is land that was previously used for industrial or commercial purposes, may have been contaminated with hazardous wastes, and is commonly found in large urban areas.
Chapter 9: Global Change
9.1: Stratospheric Ozone Depletion
Stratosphere: Contains approximately 97% of the ozone in the atmosphere, and most of it lies between 9 and 25 miles (15–40 km) above Earth’s surface.
Formation of Stratospheric Ozone
Ultraviolet radiation (uv) strikes an oxygen molecule, creating atomic oxygen.
Atomic oxygen can combine with oxygen molecules to form ozone.
Ultraviolet radiation is subdivided into three forms:
UVA: It is closest to blue light in the visible spectrum and is the form of ultraviolet radiation that usually causes skin tanning.
UVB: It causes blistering sunburns and is associated with skin cancer.
UVC: It is found only in the stratosphere and is largely responsible for the formation of ozone.
Ozone Layer: A belt of naturally occurring ozone gas that sits between 9 and 19 miles (15–30 km) above Earth and serves as a shield from the harmful ultraviolet B radiation emitted by the sun.
Ozone: A highly reactive molecule and is constantly being formed and broken down in the stratosphere.
There are no natural reservoirs of chlorofluorocarbons (CFCs) or halocarbons (halons), but their chemical stability allows them to reach the stratosphere and degrade the ozone layer.
Chlorofluorocarbons: These are nonflammable chemicals that contain atoms of carbon, chlorine, and fluorine.
Halocarbons (halons): These are organic chemical molecules that are composed of at least one carbon atom with one or more halogen atoms; the most common halogens are fluorine, chlorine, bromine, and iodine.
Effects of Ozone Depletion
A reduction in crop production
A reduction in the effectiveness of the human body’s immune system
A reduction in the growth of phytoplankton and the cumulative effect on food webs
Climatic changes
Cooling of the stratosphere
Deleterious effects on animals
Increases in cataracts
Increases in mutations, since UV radiation causes changes in the DNA structure
Increases in skin cancer
Increases in sunburns and damage to the skin
Reducing Ozone Depletion
Support legislation that reduces ozone-destroying chemicals in medical inhalers, fire extinguishers, aerosol hairsprays, wasp and hornet sprays, refrigerator and air conditioner foam insulation, and pipe insulation.
Introduce tariffs on products produced in countries that allow the use of chlorofluorocarbons (CFCs).
Offer tax credits or rebates for turning in old refrigerators and air conditioners.
Use helium, ammonia, propane, or butane as a coolant alternative to HCFCs (hydrochlorofluorocarbons) and CFCs.
9.2: The Greenhouse Effect
When sunlight strikes Earth’s surface, some of it is reflected back toward space as infrared radiation (heat).
Greenhouse gases absorb this infrared radiation and trap the heat in the atmosphere.
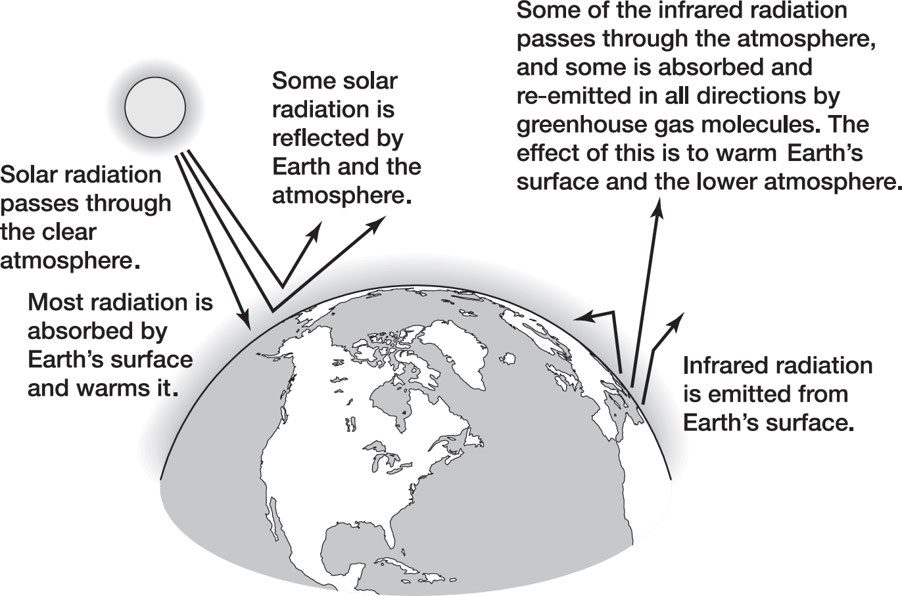
9.3: Increases in Greenhouse Gases
Greenhouse Gases by Source
Agriculture: Mostly comes from the management of agricultural soils.
Commercial and residential buildings: On-site energy generation and burning fuels for heat in buildings or cooking in homes
Energy supply: The burning of coal, natural gas, and oil for electricity and heat is the largest single source of global greenhouse gas emissions.
Industry: Primarily involves fossil fuels burned on-site at facilities for energy; cement manufacturing also contributes significant amounts of CO2 gas
Land use and forestry: It includes deforestation of old-growth forests (carbon sinks), land clearing for agriculture, strip-mining, fires, and the decay of peat soils
Transportation: It involves fossil fuels that are burned for road, rail, air, and marine transportation.
Waste and wastewater: Landfill and wastewater methane (CH4), and incineration as a method of waste management.
Greenhouse Gas Emissions by Gas
Carbon dioxide (CO2): It is an important heat-trapping (greenhouse) gas, and is released through human activities such as deforestation and burning fossil fuels, as well as natural processes such as respiration and volcanic eruptions.
Agricultural activities, waste management, and energy use all contribute to methane emissions.
Fertilizer use is the primary source of nitrous oxide emissions.
Fluorinated gases: Industrial processes, refrigeration, and the use of a variety of consumer products all contribute to this gases, which include hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6).
Black carbon (soot): It is a solid particle or aerosol, not a gas, but it also contributes to the warming of the atmosphere.
9.4: Global Climate Change
The world’s oceans contain more carbon dioxide than the atmosphere.
Atmospheric temperatures, cloud cover, surface albedo, and water vapor cause pole-wide warming.
The north and south poles are warming faster because of energy in the atmosphere that is carried to the poles through large weather systems.
Ocean currents carry heat around the Earth.
As the oceans absorb more heat from the atmosphere, sea surface temperatures rise and ocean circulation patterns change.
As the oceans store a large amount of heat, even small changes in these currents can have a large and lasting effect on the global climate.
Air temperatures today average 5°F to 9°F (3°C to 5°C) warmer than they were before the Industrial Revolution.
Higher average air temperatures may increase the frequency or severity of storms, surface water/groundwater inputs, sedimentation in bodies of water, flooding and associated water runoff, and aquifer recharge.
Global warming could completely change estuaries and coastal wetlands.
Sea-level rise threatens to inundate many coastal wetlands, threatening biota that cannot move inland due to coastal development.
The UN estimates that 150 million people will need to be relocated worldwide by 2050 due to coastal flooding, shoreline erosion, and agricultural disruption.
The total surface area of glaciers worldwide has decreased 50% since the end of the 19th century.
The main ice-covered landmass is Antarctica at the South Pole, with about 90% of the world’s ice and 70% of its freshwater.
If all of the Antarctic ice melted, sea levels around the world would rise about 200 feet (60 m).
Greenhouse gases trap solar radiation in the Earth’s atmosphere, making the climate warmer.
Due to global warming, mosquitoes have more places to breed, which increases malaria, dengue fever, Zika virus, and yellow fever rates.
Warmer water may spread amoebic dysentery, cholera, and giardia because it increases bacterial activity.
Higher air temperatures have been proven to result in higher incidences of heat-related deaths caused by cardiovascular disease, heat exhaustion, heat stroke, hyperthermia, and diabetes.
Arctic fauna will be the most affected. The food webs of polar bears that depend on ice floes, birds, and marine mammals will be drastically affected.
The movement of tectonic plates causes volcanoes and mountains to form, which can also contribute to changes in the climate
Volcanic gases that reach the stratosphere have a long-term effect on climate.
The fluctuations in the solar cycle impact Earth’s global temperature by ~0.1°, slightly hotter during solar maximums and slightly cooler during solar minimums.
As rivers and streams warm, warm-water fish are expanding into areas previously inhabited by cold-water species.
The Arctic region is a large natural source of methane.
Arctic methane release, caused by melting glaciers, creates a positive feedback loop because methane is a greenhouse gas.
Sea levels have risen 400 feet (120 m) since the peak of the last ice age approximately 18,000 years ago.
From about 13,000 years ago to the start of the Industrial Revolution, sea levels rose 0.1 to 0.2 mm per year. Since 1900, sea levels have risen about 3 mm per year.
The amount of energy absorbed and stored by the oceans has an important role in the rise of sea levels due to thermal expansion.
Ocean acidification: It occurs when atmospheric carbon dioxide reacts with seawater to form carbonic acid,
Kyoto Protocol (2005): A plan created by the United Nations to reduce the effects of climate change, which results in a reduction in the pH of ocean water over an extended period of time.
Montreal Protocol (1987): An international treaty designed to phase out the production of substances that are responsible for ozone depletion.
Paris Agreement (2016): It deals with greenhouse gas emissions and mitigation.
The goal is to keep global temperature rise below 2°C above pre-industrial levels while each country determines its own plans to mitigate global warming.
9.5: Biodiversity and Invasive Species
Plants are initially more susceptible to habitat loss than animals. This occurs for several reasons, as follows:
Plants cannot migrate.
Plants cannot seek nutrients or water.
Seedlings must survive, and they are grown in degraded conditions.
The dispersal rates of seeds are slow events
Animals can cope with habitat destruction by migration, adaptation, and/or acclimatization. Migration depends upon:
access routes or corridors;
the magnitude and rate of degradation;
the organism’s ability to migrate; and
the proximity and availability of suitable new habitats.
Adaptation: The ability to survive in changing environmental conditions.
Adaptation depends upon:
birth rate;
gene flow between populations as a function of variation;
genetic variability;
population size;
the length of generation; and
the magnitude and rate of degradation.
Acclimatization: The process by which an individual organism adjusts to a gradual change in its environment allowing it to maintain performance across a range of environmental conditions.
Acclimatization depends upon:
physiological and behavioral limitations of the species; and
the magnitude and rate of degradation.
Invasive Species
Invasive species: These are animals and plants that are transported to any area where they do not naturally live.
Characteristics of Invasive Species
Abundant in native range
Broad diet
High dispersal rates
High genetic variability
High rates of reproduction
Living in close association with humans
Long-lived
Pioneer species
Short generation times
Tolerant of a wide range of environmental conditions
Vegetative or clonal reproduction
Examples of Invasive Species
Dutch elm disease is transmitted to elm trees by elm bark beetles — killing over half of them elm trees in the northern US.
European green crabs found their way into the San Francisco Bay area in 1989 threatening commercial fisheries.
Water hyacinth is an aquatic plant, introduced to the United States from South America.
It forms dense mats, reducing sunlight for submerged plants and aquatic organisms, crowding out native aquatic plants, and clogging waterways and intake pipes.
Zebra mussels can attach to almost any hard surface—clogging water intake and discharge pipes, attaching themselves to boat hulls and docks, and even attaching to native mussels and crayfish.
9.6: Endangered Species
Endangered Species: A species considered to be facing a very high risk of extinction in the wild.
Factors are taken into account for being labeled “endangered:”
Breeding success rate
Known threats
The net increase/decrease in the population over time
The number of animals remaining in the species
Arguments for protecting endangered species
Maintaining genetic diversity
Maintaining keystone species
Maintaining indicator species
Preserving the endangered species’ aesthetic, ecological, educational, historical, recreational, and scientific value
Preserving the yet-to-be-discovered value of certain endangered species
Characteristics That Have Contributed to Endangerment
Compete for food with humans
African penguins
High infant mortality
Leatherback turtles
Highly sensitive to changes in environmental conditions
Cotton-top tamarins
Hunting for sport
Passenger pigeons, blue whales, Bengal tigers
Introduction of nonnative invasive species
Bandicoots threatened by cats that were introduced by Europeans
Limited environmental tolerance ranges
Frogs, whose eggs are sensitive to water pollution, temperature changes, and the destruction of wetlands
Limited geographic range
Pandas
Long or fixed migration routes
Salmon in the Pacific Northwest that have been driven to extinction because of dam construction, logging, and water diversion
Loss of habitat
Red wolves. Whooping cranes
Low reproductive rates
Whales, elephants, and orangutans.
Move slowly
Desert tortoises
No natural predators, which makes them vulnerable as they lack natural defensive behaviors and mechanisms
Dodo birds, Steller’s sea cows, sea otters
Not able to adapt quickly
Polar bears
Possess characteristics sought after for commercial purposes
Sharks, elephants, rhinoceros’ horns. gorillas
Require large amounts of territory
Tigers
Small numbers of the species, which limits genetic diversity
Tigers
Specialized feeding behaviors and/or diet
Pandas (Bamboo)
Spread of disease by humans or livestock
African wild dogs
Superstitions
Aye ayes—some people native to Madagascar believe that aye ayes bring bad luck, and therefore kill them.
Maintaining Biodiversity
Creating and expanding wildlife sanctuaries
Establishing breeding programs for endangered or threatened species
Managing habitats and monitoring land use
Properly designing and updating laws that legally protect endangered and threatened species.
Protecting the habitats of endangered species through private and/or governmental land trusts
Reintroducing species into suitable habitats
Restoring compromised ecosystems
Reducing nonnative and invasive species
 Knowt
Knowt
