DEN1003 Renal Regulation of H+
LO1: Define the normal range for the pH of body fluids
Normal body fluid pH range: 7.35 - 7.45
LO2: Explain why it is important to maintain the pH of body fluids within these limits
Metabolism of nutrients produces acidic byproducts
Deviations from normal pH can disrupt enzyme function and metabolism
Mechanisms must be in place to resist changes in pH, focusing on kidney functions.
LO3: Understand the difference between chemical and physiological buffers
Chemical Buffers:
Bind/remove H+ as solution acidity changes.
Present in all body fluids and act within seconds (first line of defence)
2 types:
Protein Buffer System:
Proteins provide ¾ of chemical buffering in ICF and ECF through carboxyl and amino groups.
Bicarbonate Buffer System:
Also present in ICF and ECF and is important for pH regulation via kidneys; forms carbonic acid (H2CO3) from CO2 and H2O, in the presence of carbonic anhydrase
Reaction: CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3-
HCO3- acts as a base in acidic conditions (by combining with H+) and as an acid in basic conditions (by dissociating into H+ and HCO3-).
Physiological Buffers:
Stabilise pH by controlling acid/base or volatile acid excretion (CO2) through renal and respiratory systems, respectively.
Physio buffers have a greater buffering capacity than chemical buffers
Renal system has the greatest buffering capacity; takes hours to cause changes.
Respiratory system has less capacity but responds quickly (minutes).
LO4: Describe how the renal system contributes to the regulation of body fluid pH
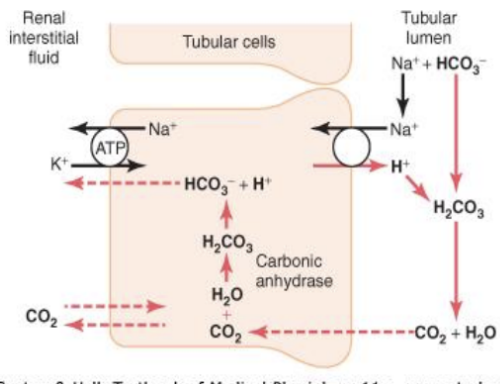
Kidneys regulate body fluid pH by controlling the excretion of H+ and reabsorption of HCO3- ions.
HCO3- is filtered from plasma into tubular fluid at the glomerulus
Inside tubular cells: CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3− (in the presence of carbonic anhydrase)
The H+ is secreted into the tubular fluid via the Na+/H+ exchanger, which reacts with the filtered HCO3- in the tubular fluid: H+ + HCO3− ⇌ H2CO3 ⇌ CO2 + H2O
CO2 and H2O from the tubular fluid and CO2 from the interstitial fluid diffuse into tubular cells to produce HCO3- and H+ via the bicarbonate buffer system reaction again
The HCO3- ions diffuse across the tubular cell membrane into the interstitial fluid to enter the peritubular capillaries.
For every H+ secreted, a HCO3- enters the blood, so H+ is lost but HCO3- is not → explaining why at a normal body fluid pH, urine produced is slightly acidic
LO5: Describe the conditions of acidosis and alkalosis
Acidosis: pH < 7.35
Causes cardiovascular dysfunction and CNS depression that leads to confusion and seizures, but can lead to coma followed by death if pH falls <7.0.
Types:
Respiratory Acidosis: CO2 builds up which increases [H+], lowering pH.
Metabolic Acidosis: Low pH not caused by CO2 excess.
It’s because [H+] in ECF (which includes the renal ISF) increases, causing reaction in tubular cells: CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3− to shift left, ↑ CO2 in the renal ISF
more CO2 diffuses into tubular cells down a conc. gradient, increasing H2CO3 production in tubular cells
↑ H2CO3 leads to more dissociation into H+, so more H+ secreted by the Na+,H+ exchanger (Since for every H+ secreted, a HCO3- is reabsorbed, more HCO3- is reabsorbed)
To correct this, less HCO3- is excreted/more reabsorbed: CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3−, [HCO3-] ↑, shifting the reaction right to decrease [H+]
Alkalosis: pH > 7.45
Symptoms may include confusion, spontaneous skeleteal muscle spasms, tetany, and respiratory paralysis.
Types:
Respiratory Alkalosis: Caused by CO2 deficiency which lowers [H+], increasing pH.
Metabolic Alkalosis: Low pH not caused by CO2 excess.
It’s because [H+] in ECF (which includes the renal ISF) decreases, causing reaction in tubular cells: CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3− to shift right, ↓ CO2 in the renal ISF
less CO2 available to diffuse into tubular cells, decreasing H2CO3 production in tubular cells
↓ H2CO3 leads to less dissociation into H+, so less H+ secreted by the Na+,H+ exchanger (Since for every H+ secreted, a HCO3- is reabsorbed, less HCO3- is reabsorbed)
To correct this, more HCO3- is excreted/less reabsorbed: CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3−, [HCO3-] ↓, shifting the reaction left to increase [H+]
LO6: Explain the role of ammonia in buffering H+ excretion in the urine
H+ excretion depends on the conc. gradient between tubular cells and tubular fluid in the tubular lumen (↑ [H+ in tubular cells: high pH, ↓[H+] in tubular fluid in lumen: low pH)
If the pH of the tubular fluid falls below 4.5, the concentration gradient is not sufficient for H+ excretion, so excess H+ reacts with NH3 to form NH4Cl
LO7: Describe the involuntary and voluntary components of urination (micturition)
Involuntary Component (Micturition Reflex):
When the bladder contains about 200 ml urine, stretch receptors in the bladder send signals to the sacral region of the spinal cord via pelvic nerves.
Spinal cord reflex sends signals via the same pelvic nerves to cause contraction of the bladder detrusor muscle and relaxation of the internal urethral sphincter.
This reflex empties the bladders of infants and young children until voluntary control of the external urethral sphincter is achieved due to development of somatic fibres which innervate it.
Voluntary Control:
Sensory signals to to the sacral region of the spinal cord also stimulate the micturation centre in the pons, which integrates info from stretch receptors with info from the cerebrum (decision making centre) to establish the appropriateness of urinating at that time
If not appropriate: the pons inhibits the micturation reflex (detrusor muscle contraction and internal sphincter relaxation). It increases the frequency and intensity of impulses to the external urethral sphincter via the pudenal nerves to to keep it closed. However, if voluntary urination is not permitted, the sheer force of the fluid against the bladder wall will force open the external urethral sphincter, causing urination
If appropriate: the pons induces the micturation relfex (detrusor muscle contraction and internal sphincter relaxation). It decreases the frequency and intensity of impulses to the external urethral sphincter via the pudenal nerves to open it, causing urine to flow down the urethra and out of the body.