Organic Chemistry
Organic family: a group of organic compounds that have common chemical, structural and physical features
Functional group: cluster of atoms that bring chemical, structural and physical properties
Hydrocarbons are organic molecules containing only carbon and oxygen; there can be organic derivatives like alkyl halides, alcohols, ketones, aldehydes, carboxylic acids…
Structural isomers: have the same molecular formula but different connectivity of the carbon backbone.
Number of Carbons | Prefix |
|---|---|
one | meth- |
two | eth- |
three | prop- |
four | but- |
five | pent- |
six | hex- |
seven | hept- |
eight | oct- |
nine | non- |
ten | dec- |
eleven | undeca- |
General Naming
With the exception of carbonyl groups such as ketones and aldehydes, the alcohol or hydroxy groups have first priority for naming.
Numerically indicate the position of double/triple bonds
Cyclic hydrocarbons: numerical locators are required if there are two or more groups
All numerals are separated by commas; numerals and letters are separated by hyphens; and names of branches and parent chains are not separated.
di,tri-> not part of alphabetical ordering
The three main hydrocarbons are:
Alkanes:
Saturated hydrocarbons contain only single (sigma) bonds.
Because of their single bonds, they are hard to break and are mainly unreactive.
That said, they can undergo combustion reactions when ignited in the air.
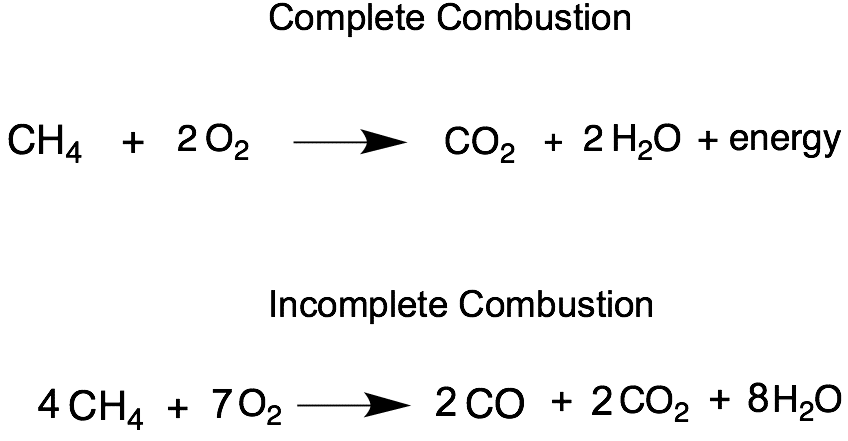
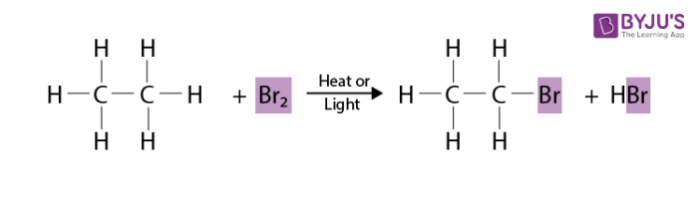
Their hydrogen can also undergo substitution reactions with a halogen in the presence of heat or light to dissociate the diatomic halogen, resulting in a haloalkane and a halogen bonded to a hydrogen atom.
To name an alkane, check the number of carbons present and choose an according prefix, then change the ending to “-ane.”
Alkenes and Alkynes:
Alkenes contain one or more carbon-carbon double bonds (one pi bond and one sigma bond)
Alkynes have one or more carbon-carbon triple bonds (two pi bonds and one sigma bond)
Because of the presence of the pi bonds, they are easier to break and are more reactive than alkanes
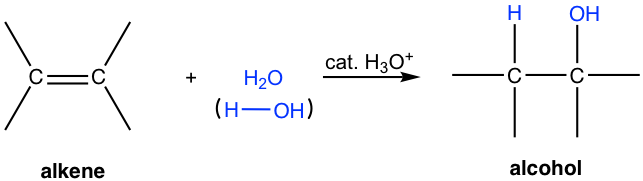
Alkenes can undergo additional reactions with water under the presence of acid to form alcohols:
They can also undergo halogenation with a halogen bonded to an H at room temperature to form an alkyl halide:

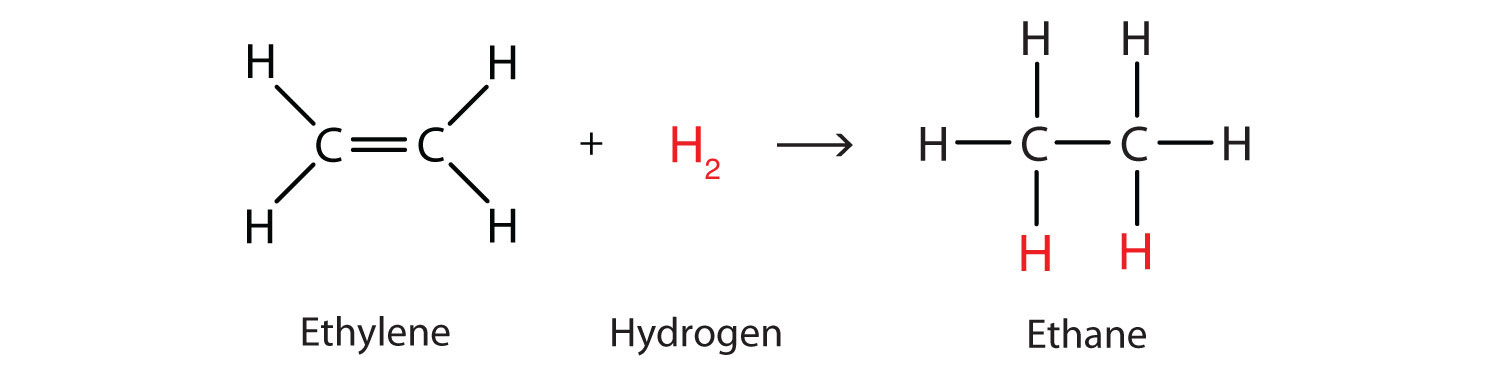
Furthermore, they can also undergo an addition reaction with diatomic hydrogen under the presence of heat, pressure and a catalyst to become alkanes:
To name Alkenes, check the number of carbons present and choose an according prefix, then change the ending to “-ene.” - if there is more than one double bond, add “di” (2) or “tri” (3) before the “-one” ending.
To name Alkynes, check the number of carbons present and choose an according prefix, then change the ending to “-one.”- if there is more than one double bond, add “di” (2) or “tri” (3) before the “-one” ending.
Markonikov’s rule states that “the rich get richer and the poor get poorer.” - The carbons with the most hydrogens before the reaction are most likely to gain the hydrogen after the response (addition), and the carbon with the least hydrogens. Before the reaction, it is most likely to lose hydrogens after the reaction (elimination)
Aromatic hydrocarbons:
Aromatic hydrocarbons are all based on benzene, a flat, 6-carbon ring with delocalized pi bonds:
When benzene is a functional group, it takes the name “phenyl.”
Aromatic hydrocarbons are less reactive than alkenes but more reactive than alkanes
They can undergo substitution reactions requiring heat and catalysts and addition reactions at room temperature.
Benzene cannot undergo addition reactions
To name cyclic hydrocarbons, add “cyclo-” in front of the suffix
The chair conformations are the most stable arrangement cyclohexane can form.
Every other atom lies in the same plane
Most stable as it ensures bond angles are as close to 109.5 degrees as possible
Angle more appropriate to hybridization (tetrahedral —> sp3)
Constantly shifting when energized
Uses that energy to get the most stable arrangement
axial bonds are positioned perpendicular (straight) to the structure and are shown in red. Meanwhile, equatorial bonds stand slanted and are shown in blue
As your ring becomes smaller, the bond angles become smaller, experiencing more tension
Properties of hydrocarbons:
Hydrocarbons only rely on weak, temporary LDF forces. They are insoluble in water (nonpolar)
Alkanes are unreactive due to their sigma bond, causing higher boiling points than alkene, alkynes and cyclic hydrocarbons.
Aromatic hydrocarbons induce more LDF than alkanes due to their increase in surface area. - higher mp/bp than alkenes and alkynes
Alkenes have a higher mp/bp than alkynes because they only have one pi bond.
Organic Halides
They are derivatives of hydrocarbons with one or more halogens bonded.
Alkyl halides come from the substitution of halogens and alkanes or the addition of halogens and alkynes/alkenes.
They can undergo elimination reactions with a basic catalyst to form alkenes/ alkynes.
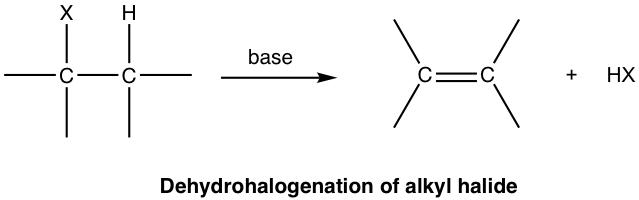
To name alkyl halides, locate the halogen on the carbon, counting from the side closest to the halogen and add a numeric locator with a hyphen following. Shorten the name of the halogen (chloro,bromo,iodo…). Then, choose the name of the alkyl best suited and add the ending “-ane” if it is an alkane or “-ene” if it is an alkene. I
If multiple of the same halogen bonded, add “di” or “tri” in front of the halogen’s name
i.e. 1,4-dichlorobutane
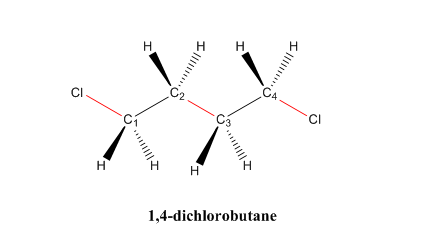
If multiple halogens bonded, the one that comes first in alphabetical order comes first in the name.
Because of the presence of the halogen, alkyl halides have the ability to strong, permanent dipole-dipole forces
gets more vital as the electronegative value of the halogen increases
They have higher mp/bp than hydrocarbons
The halogen also makes the hydrocarbon polar, which makes them soluble in water.
The longer the hydrocarbon, the less soluble it becomes because the significance of the halogen diminishes.
Alcohols:
To name alcohols, change the ending of the parent chain to “-ol.”
If multiple alcohols are attached, add “-diol” or “triol.”
i.e. 1 3-butanediol
The hydroxyl group has a numbering priority over alkyl halides and hydrocarbons.
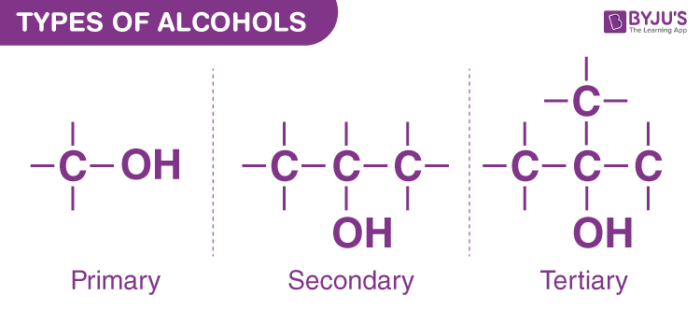
Primary alcohols have the hydroxyl group on the first carbon
Secondary alcohols have the hydroxyl group on the second carbon
Tertiary alcohols have the hydroxyl group on the third carbon
Alcohols come from the addition reaction of water and alkenes with the presence of acid
Alcohols can undergo combustion reaction to dissociate:
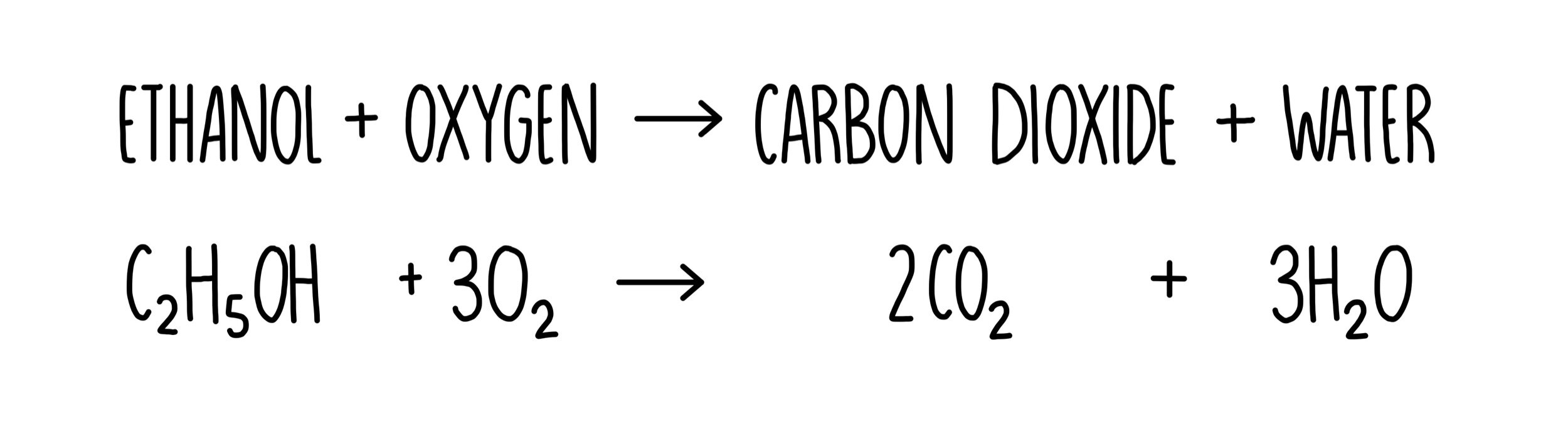
Alcohols can also undergo elimination reactions under the presence of a catalyst to form alkenes
The hydroxyl group of alcohols enables the ability to form H-bonds - making it have much higher boiling points than alkyl halides and hydrocarbons.
The oxygen makes the alcohol highly polar and enables it to dissolve in polar compounds like water, much better than alkyl halides.
The longer the hydrocarbon, the less polar and lower the mp/bp it becomes because the significance of the hydroxyl group diminishes.
Ethers
made from the condensation reaction of two alcohols in the presence of a catalyst
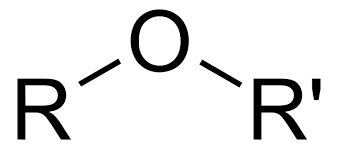
Ethers lose h-bond ability due to the lack of the hydroxyl groups, but polarity remains.
Intermolecular forces are more potent than hydrocarbon and alkyl halides but weaker than alcohols.
C-O-C is hard to break
To name ether and “-oxy” to the ending of the smaller carbon chain and keep the name of the longer chain the same.
Ether has numbering priority over halogens and hydrocarbons but not hydroxy.
i.e. ethoxypropane
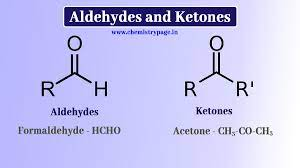
Aldehydes & Keytones
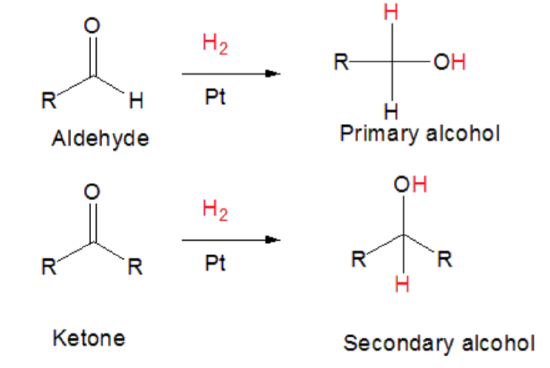
Aldehydes are made from the oxidation reaction of primary alcohols
Ketones are made from the oxidation of secondary alcohols
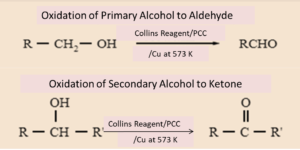
Tertiary alcohols cannot undergo oxidation - no h atoms available on central C
Aldehydes have their carbonyl group on the outer carbons and have the suffix “-al.”
Ketones have their carbonyl group on the inner carbons and have a suffix “-none.
Both Ketones and Aldehydes have lower mp/bp than alcohols and ethers but still have the polarity from the oxygen to have dipole-dipole forces - soluble in water.
As the number of carbon increases, the dipole-dipole forces and solubility diminishes.
As the carbonyl group moves down the carbon chain, it disrupts the surface area and limits chain interactions, reducing LDF and boiling/melting points.
Aldehydes and ketones can undergo addition (hydronation) reactions with a diatomic H2 atom to form primary or secondary alcohols under heat, pressure and a catalyst.
Carboxylic acids + ester
Carboxylic acids are made from the oxidation reaction of aldehydes.
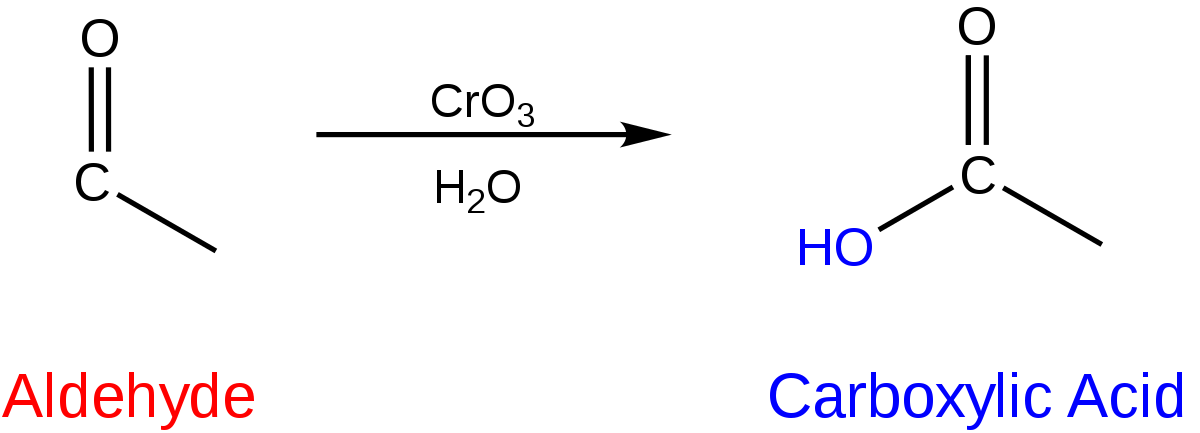
The ending of the parent chain changes to “-oic acid.”
When there are two carboxylic groups, add “-dioic acid” as the ending.
The parent chain is the longest continuous chain containing a carbon double bonded to oxygen.
Because Carboxylic acids have a carbonyl and hydroxyl group, they are highly polar and can induce strong, permanent dipole-dipole forces as well as H-bonds
They have higher solubility in water, mp and bp than alcohols, ethers, alkyl halides, and hydrocarbons.
The longer the hydrocarbon chain, the lower the solubility and mp/bp of the carboxylic acid due to the significance of the carboxyl group diminishing.
Esters are made from the condensation reaction of a carboxylic acid and an alcohol.
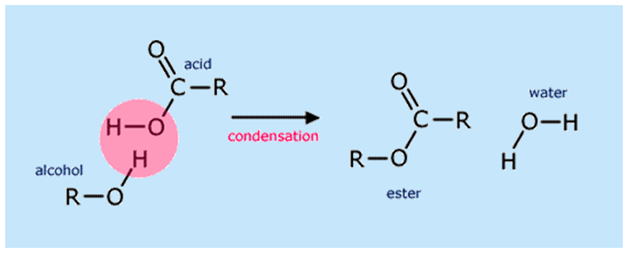
The naming of ester has two parts:
The smaller chain (which does not contain C- -O has the ending “yl”
i.e. methyl, ethyl
The longer chain (containing C- -O) ends with the suffix “-oate”
Esters lose their H-bonds capacities and therefore are less soluble in water and have lower mp/bp than carboxylic acids and alcohols.
They are more soluble and have higher mp/bps than others due to having two O atoms
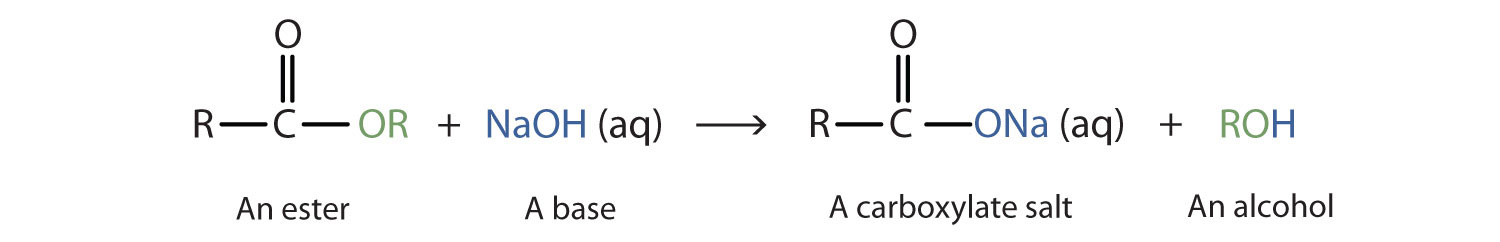
Esters can perform hydrolysis to create a carboxylic acid and an alcohol with the presence of a basic catalyst.
Amines and Amides
Both amines and amides are derivatives of ammonia.
Primary amines are made from the substitution reaction of alkyl halides and ammonia
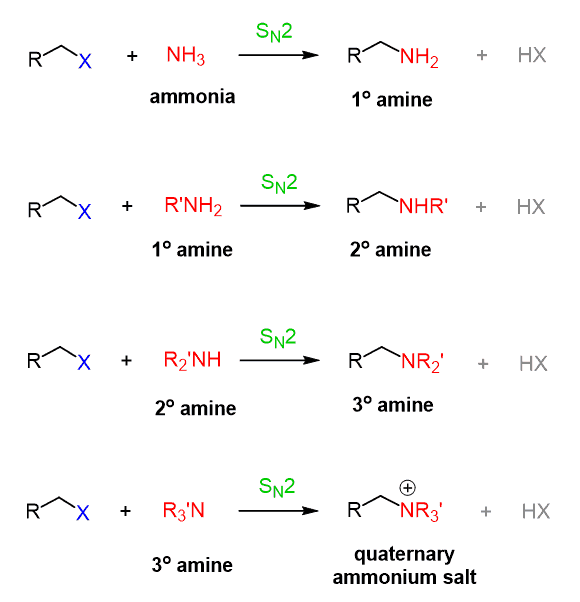
Primary amines can synthesize further with ammonia to make secondary amines.
When one of the H on ammonia is replaced by a hydrocarbon, it is a primary amine.
When two of the H on ammonia are replaced by a hydrocarbon, it is a secondary amine.
When three of the H on ammonia is replaced by a hydrocarbon, it is a tertiary amine.
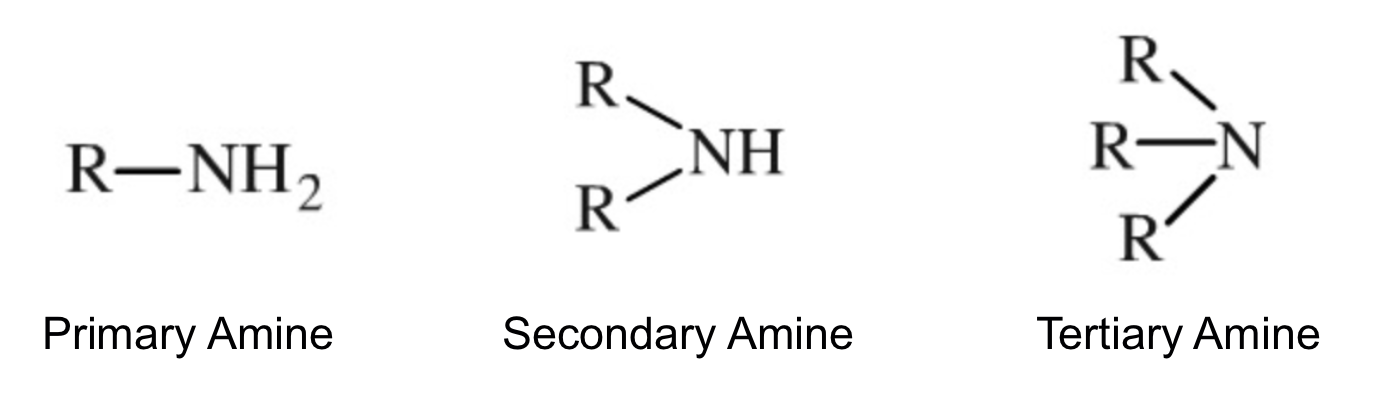
Primary amines have stronger intermolecular forces than the rest because there are two hydrogens bonded to the nitrogen, making the H-bonds more prominent.
To name amines, change the name of the parent chain, longest chain to “-amine.”
i.e. methylamine, ethylamine
When its a secondary amine, add “N” followed by a hyphen in front of the name of the shortest chain (if the chains are different)
If the chains are the same on secondary or tertiary amines, just change the ending to “-diamine” or “triamine”
i.e. Dimethylamine, Triethylamine
Amines have higher mp/bp and solubility in water than hydrocarbons, esters, ether, aldehydes, and ketones…but lower than carboxylic acids, and alcohols - because N has a lower EN value than Oxygen, less polar.
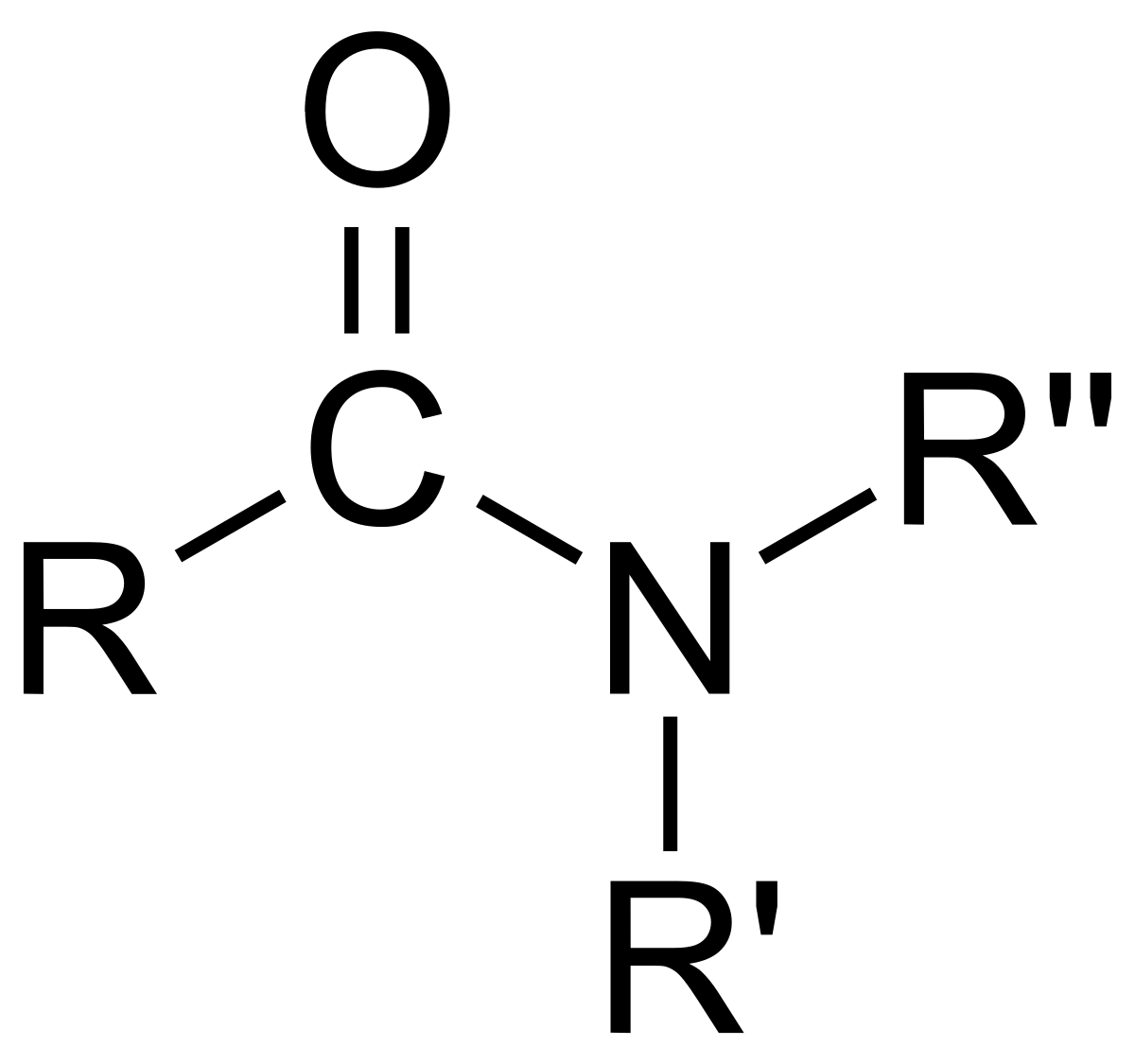
Amides are similar to esters, but the oxygen bonding of the two hydrocarbons is replaced by N.
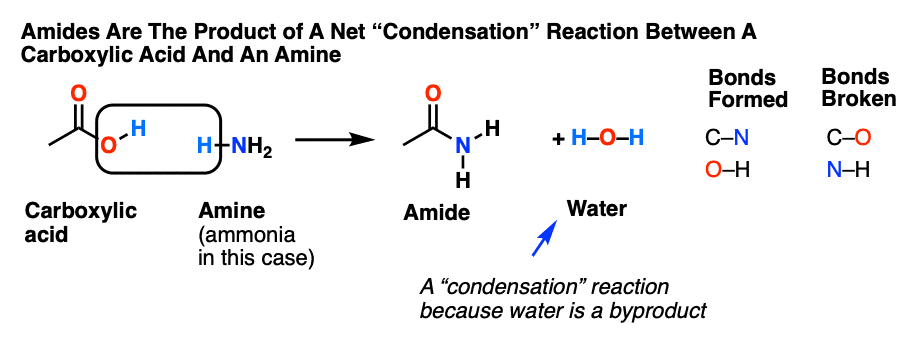
Amides are created from the condensation reaction of a carboxylic acid and an amine
Tertiary amines cannot undergo substitution reactions
To name esters, change the ending of the parent chain (chain with C- - O ) to “-amide”. When an alkyl group is attached, add “N,” followed by a hyphen in front of the alky’s name.
Amides can undergo hydrolysis with an acid catalyst to form a carboxylic acid and an amine.
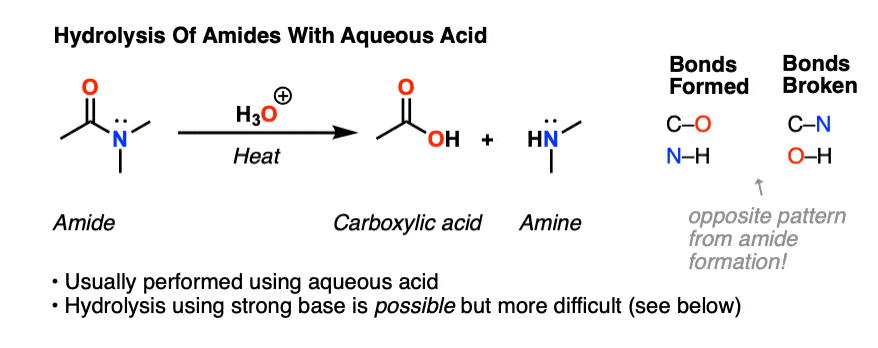
Amides have the highest boiling and melting points of all of the other organic families.
So…
Amides>Carboxylic acids> alcohols>esters>ethers>aldehydes>ketones>alkyl halides> C-C>aromatic hydrocarbons>C- - C> C- - -C