Chemistry IB SL Acids and Bases
Theory of acids and bases
The role of acids and bases
Acids and bases are opposites
Many theories describe how they work
Arrhenius Theory of acids and bases
Acids produce H+ ions, bases produce OH– ions
Neutralization is the process of combining an acid and a base
Limitation: weak base ammonia and hydrogen chloride gas could not be explained, as ammonia does not contain OH–
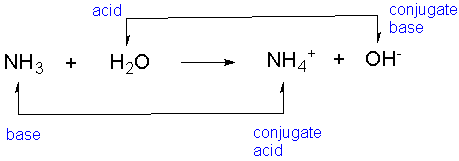
Bronsted-Lowry acids and bases
Refer to hydrogen ion as a proton;
Acid is proton donor, base is proton acceptor
Conjugate acids and bases
In a reversible reaction between acid and bases, there are conjugates as well
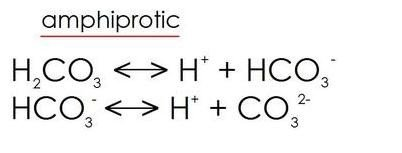
Amphiprotic species
Amphiprotic species: Substances which can be both Bronsted-Lowry acids or bases depending on the reaction
Examples:
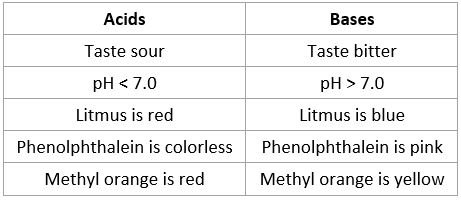
Properties of acids and bases
Properties of acids and bases
Reactions of acids with metals, bases, and carbonates
Most acids react with metals, metal oxides, hydroxides, hydrogencarbonates and carbonates
All these reactions produce a salt which is a compound of anion and cation
Standard enthalpy change of neutralization: the energy change associate with the formation of 1 mol of water from the reaction between a strong acid and a strong base under standard conditions
Negative value because neutralization is exothermic
For acids and bases:
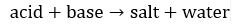
For metals higher than hydrogen on activities series:
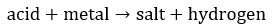
For metal carbonates and hydrogencarbonates:
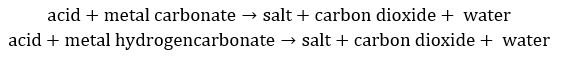
Acid-Base titrations
Refer to section 22 of data booklet to see colour changes associate with indicators
The pH scale
The pH scale
pH scale is effective way of representing concentration of hydrogen ions [H+] in a solution
It is a logarithmic scale with base 10, easy way for non-scientist to understand safety of materials
pH distinguishes between acidic, neutral and alkaline
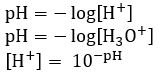
Calculating pH
The concentration of an acid with one proton is the same as the concentration of hydrogen ions [H+]
[HCl] = [H+]
For more protons:
[H2SO4] = 2[H+]
Ionization of water
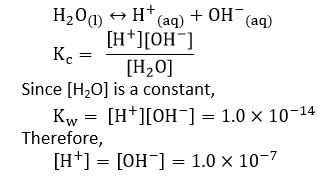
Strong and weak acids and bases
Strengths of acids and bases
Strength of an acid or base depends on the degree to which it ionizes (dissociates) in water
In the following reactions, an acid or a base reacts with water to produce conjugates
Strong acid is an effective proton donor that is assumed to completely dissociate in water.
Examples: HCl, H2SO4 and HNO3
Conjugate base of strong acid is weak base
Weak acid only partially dissociates in water; it is a poor proton donor.
Examples: CH3COOH and H2CO3
The dissociation of a weak acid is a reversible reaction that reaches equilibrium
Only small portion of acid molecules have dissociated at equilibrium
Conjugate base of weak acid is strong base
Strong base completely dissociates in water
Examples: KOH, NaOH (Any group 1 metal hydroxides)
Note: Metal hydroxides don’t act as Bronsted-Lowry bases, however in solution the hydroxide ion OH– acts as a base
Weak base partially dissociates in water
Example: NH3
Words strong and concentrated, and weak and diluted have very different meanings.
Strong: completely dissociated into ions
Concentrated: a high number of moles of solute per litre of solution
Weak: slightly dissociated
Diluted: a low number of moles of solute per litre of solution
Experimental determination of the strength of acids and bases
Techniques to compare strength of acids and bases of equal concentration
Conductivity
All acids and bases dissociate in water to form ions. The conductivity of an aqueous solution depends on the concentration of ions present. Voltage is applied to different solutions (of equal concentration), and the voltage reflects on concentration of ions, and thus strength as well.
Strong acids and bases have higher conductivities
Weak acids and bases have lower conductivities
Energy changes on neutralization
Neutralization: occurs when an acid and base react together
This reaction is exothermic, ΔH < 0
The enthalpy change of neutralization for a strong acid is almost identical to that of weak acid
For strong acid or base:
They are completely dissociated in water, thus only enthalpy change to consider is exothermic formation of water from H+ and OH– ions
For weak acid or base:
Dissociation of weak acid or base is mildly endothermic
Enthalpy change for strong base-weak acid is less than strong base-strong acid
The weaker the acid, the more endothermic, and thus lower enthalpy change of neutralization
Acid deposition
Acid deposition: the process by which acid-forming pollutants are deposited on the earth’s surface.
Increased industrialization have led to rapidly increasing emission of nitrogen and sulfur dioxides which cause acid rain, most prevalent form of acid deposition
Acid rain
Pure water has pH of 7.0. Rainwater is acidic due to presence of dissolved carbon dioxide which forms weak carbonic acid. Typical pH is 5.6
Acid deposition is formed when nitrogen or sulfur oxides dissolve in water to form HNO3, HNO2, H2SO4 and H2SO3
The sources of nitrogen and sulfur are:
Volcanic eruptions
Decomposition of vegetation
Combustion of fossil fuels
Go over nitrogen and sulfur cycles which form the acids
Pre- and post-combustion technologies
Pre-combustion methods: refer to techniques used on fuels before their combustion
Post-combustion methods: focus on several complementary technologies to remove sulfur dioxide, nitrogen oxides and other gases from combustion gases before they are released into the atmosphere.
How to find parent acid
Alkalis
Soluble bases that release an OH- ion when they react with water: K2O(s) + H2O(l) → 2K+(aq) + 2OH–(aq)
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH–(aq)
CO32–(aq) + H2O(l) ⇌ HCO3–(aq) + OH–(aq)
HCO3–(aq) ⇌ CO2(g) + OH–(aq)
Note: All alkalis are bases, but not all bases are alkalis
Acid Reaction Types (p 351-353)
Simplest: Acids react with bases to form water + a salt (an ionic compound)
1) Acid + Metal → Salt + Hydrogen gas
Samples:
2) Acid + Base → Salt + Water
Samples: