3.1G Colored Complexes
Refresher
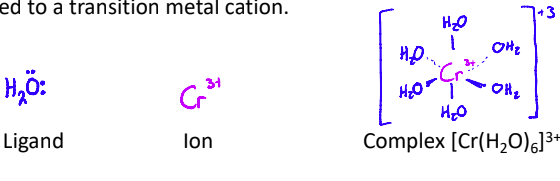
Transition elements form coloured compounds known as complexes when bonded with ligands.
Ligands are molecules or ions with a lone pair of electrons that can be donated to a transition metal cation.
 Splitting of d-orbitals
Splitting of d-orbitals
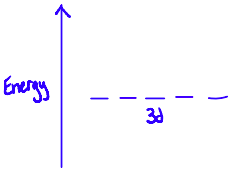
When there are no ligands, the d-orbitals of transition metal cations are degenerate. This means that they are all equal in energy
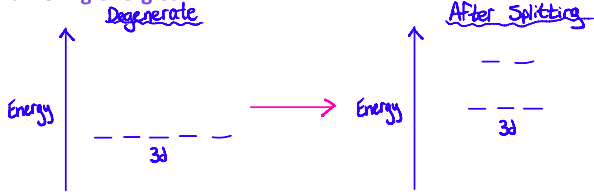
When ligands approach a metal ion, repulsion between the lone pairs of electrons on the ligand and electrons in the d-orbitals of the transition metal cause the d-orbitals to be split into two sets of differing energies
 Absorbance of Coloured Light
Absorbance of Coloured Light
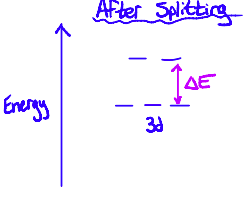
The differences in energy between the split orbitals allows electrons in the lower energy orbitals to be promoted into the higher energy orbitals. This happens when the complex absorbs light with a wavelength that corresponds with the energy gap.
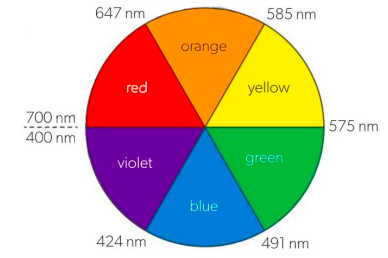
 The observed colour is complementary to the light that is absorbed
The observed colour is complementary to the light that is absorbed
If red light is absorbed, the complex will appear green.
If yellow light is absorbed, the complex will appear purple.
If blue light is absorbed, the complex will appear orange
 c = fλ
c = fλ
E = hf
Using these two equations, we can calculate the wavelength of light that would be absorbed for an energy gap (or the other way around)
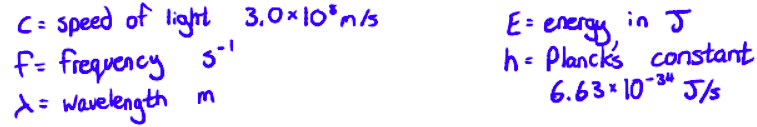 Factors Affecting Colour
Factors Affecting Colour
The identities of the ligand and the transition element cation both affect the colour of the complex observed.
Ligands that form stronger coordination bonds with the central ion will produce a greater gap between the split d-orbitals.