Thermodynamic & Equilibrium
Dilutions reduce the concentration. The more dilute a solution, the greater the percent ionization.
Driving forces:
Enthalpy: the measure of bond stability in a system
systems favour lower enthalpy (more stable)
Units —> Kilojules/mole
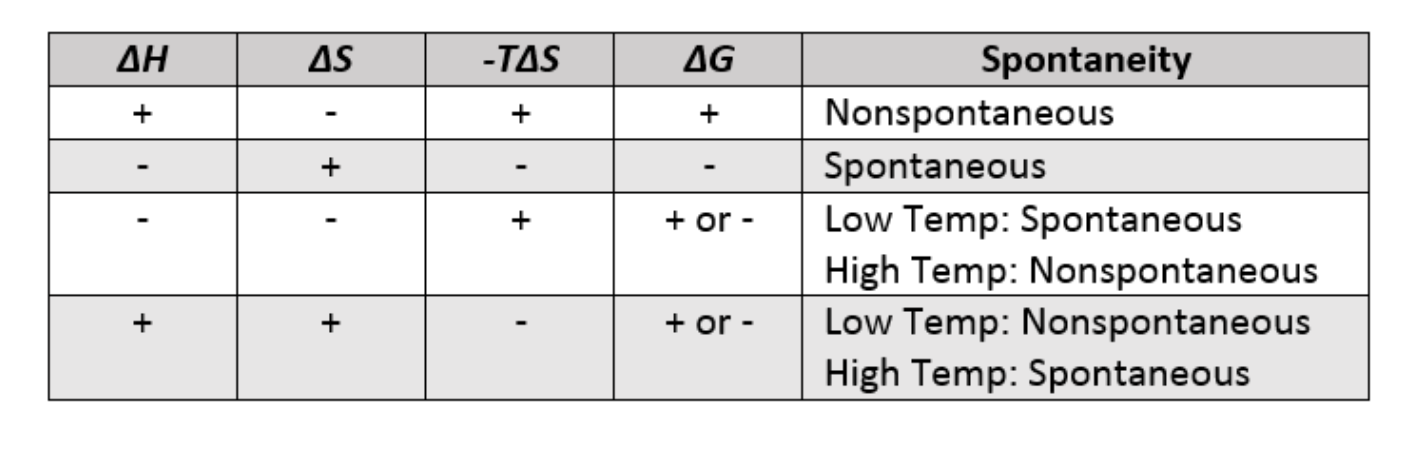
Exothermic - releases energy to surroundings, spontaneous (-ΔH) - + energy on the product side
Endothermic - gains energy from surroundings, nonspontaneous (+ΔH) - + energy on the reactant side
Spontaneous - starts without continuous outside influence
Entropy: The measure of disorder in a system
systems tend to favour higher entropy (more disorder)
More gas moles increase entropy
Solid - very little entropy
aqueous/liquid - somewhat entropy
gas - high entropy
At higher temperature, there is more entropy
Gain in disorder = +ΔS
units —> Jules/Kelvin
Bond energy: the minimum energy required to break one of the bonds between 2 particular atoms - stability of a covalent bond
only in gase systems
To calculate bond energy, add up the bond energy value and multiply by the moles of reactants, then subtract by that of the products.
Units —> Kilojoules
Free energy: energy that is available to do work
ΔG° = ΔH° - TΔS°.
Units —> Kilojoules
When ΔG is positive, it is nonspontaneous (reverse direction) - low solubility
when it is negative, it is spontaneous (thermodynamically favoured) - provides energy to surroundings (forward direction) - high solubility
ΔG = 0, the system is at equilibrium
Dynamic equilibrium:
at dynamic equilibrium, the reverse rate and forward rates are equal; Rr=Rf
no net gain or loss of energy
optimizes system
The percent reaction always refers to the amount of products formed in the reaction
%reaction = (actual yield/theoretical yield) x 100.
%<1%, strongly reactant favoured
%>99%, strongly product favoured
1%<%>99%, equilibrium
less than 50% reactant favoured
more than 50% of products favoured
An ICE (Initial, Change, Equilibrium) table is a convenient way of organizing info
the change in concentration depends on the molar coefficient of the molecule.
Le Chatelier Principle:
when a system at equilibrium is disturbed by a change in property, it adjusts in a way that opposes the change
the system undergoes an equilibrium shift
When an equilibrium shift occurs, the systems are no longer at equilibrium ( reverse rate does not equal forward rate, but eventually, the two rates become equal to each other again, and equilibrium is restored
the addition or removal of liquid or solid states does not change the concentration of molecules in the system - no shifts
Energy can be added or removed from systems by heating or cooling
when a system is cooled (loses energy), it shifts in the direction that produces heat- where energy is
when a system is heated (gain energy), it shifts in the direction that absorbs heat.
when cooling exothermic systems, both the reverse and forward rates are slow at low temps, but the reverse rate decreases more than the forward rate ( production of more products) - Rf>Rr. The shift causes concentration rates that will increase the reverse rate and make both rate equal again at the new low temp
As the volume in a system is increased, the pressure (amount of gas molecules) decreases
If the volume is increased, the system shifts in the direction with the most gas moles
if the volume is decreased, the system shifts in the direction with the least gas moles
Adding catalyst or inert gases (noble gas, or gas that does not react with system) does not affect the equilibrium shift.
Whenever a stress is placed on a system, the reaction will shift to go back to equilibrium. If a reaction shifts left, it will create more reactants. If it shifts right, it will make more products.
Concentration
If concentration is altered, the reaction will shift to use up more or less of the substance with an altered concentration.
For the Haber process, if N2 or H2 is added, it will shift right to create more NH3 and use up more of the reactants. If more NH3 is added, the reaction will shift left to create more N2 and H2.
If N2 or H2 is removed, it will shift left to make up for the loss of the reactant and create more reactants.
Pressure
When external pressure is increased, the partial pressure of gases inside a container will increase, and the reaction will shift to the side with less gas molecules. Decreasing the partial pressure will cause the reaction to shift to the side with more gas particles
If the pressure of a container is increased, the reaction will shift to the right where there are 2 moles of gaseous products rather then the left, where there are four moles of gaseous reactants.
If the pressure is decreased, the reaction will shift left where there’s more moles of gas.
If a noble gas is added at a constant pressure, the equilibrium reaction will shift to the side with more gaseous molecules. If a noble gas is added to a reaction at a constant volume, no shift occurs.
Temperature
The Haber process has a ΔH of -92.6 kj/mol, so it is exothermic, so heat is generated, this creates the equation:
N2+3H2 ⇌ 2NH3 + energy
If the temperature goes up, the reaction will shift to the left, making more reactants. If the temperature goes down, the reaction would shift to the right and produce more products
Dilution
If an aqueous equilibrium is diluted, such as the equation Fe 3+ (aq) + SCN- (aq) ⇌ FeSCN 2+ (aq), diluting an equation with extra water will cause a shift.
If this equation were diluted, it would shift the side with more aqueous molecules to the left. If water evaporated, it would shift to the side with less aqueous molecules, or the right.
Solubility product constant
A special equilibrium may be where excess solutes is in equilibrium with an aqueous solution
the solubility equilibrium law equations must be: Ksp= [products]/[reactants]
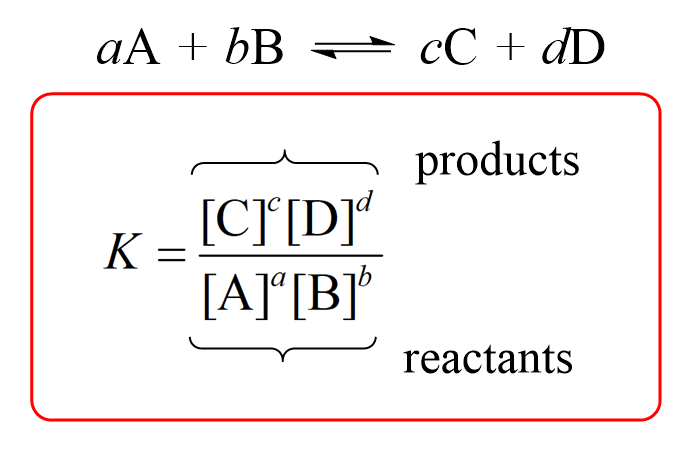
Solids are not taken into consideration for ICE table and Ksp calculations because their concentration is always constant
small Ksp values indicate low solubility
The reaction quotient, Q, can be used to predict a precipitate
to predict whether or not a precipitate will form, compare the Ksp to the Q
Q<Ksp —> no precipitate occurs, unsaturated
Q=Ksp —> no precipitate occurs, saturated
Q<Ksp —> precipitate will form, supersaturated
The Common Ion Effect
If AgCl is put into a solution to dissolve, it will only do so in small amounts. If NaCl is added to the solution, it will dissolve completely and not affect the Ag ions. The Na ions can be ignored, but all of the Cl ions from the NaCl and AgCl must be considered because of the common ion effect.
According to the common ion effect, the new Cl ions will affect the AgCl equilibrium even though the Cl ions came from NaCl.
The common ion effect reduces the solubility of a salt
Acids and Bases
A single arrow represents a strong acid/base
When a stong acid reacts with water, the hydrogen ion is transferred to the water to form a hydronium ion and a conjugate base for the acid
When double arrows are present, it signifies a weak base/acid
Strong acids have more than 99.9% ionization (completely ionized)
a strong acid has a fragile attraction for its protein (H+), making it easier to donate to water.
A strong base had a very strong attraction for its proton
weak acids have less than 50% ionization (partially ionized)
The stronger the acid, the weaker its conjugate base
An acid is a proton donor, and a base is a proton acceptor
Water can act both as a base and an acid (amphoteric)
Only some hydrogens in an acid are able to dissociate
This is why acetic acid is written as HC2H3O2 rather than C2H4O2
The hydrogen will dissociate from the least electronegative end.
However hydrogens attached to a carbon nearly never dissociate because they share the electron fairly equally because of the similar electronegativity.
Generally, hydrogens that are written in the beginning of an acid are able to dissociate while ones in the middle cannot
In a proton transfer reaction, both the forward and reverse reactions involve B-L acids and bases
A pair of substances whose molecular formula differs by a single H+ ion is called a conjugate acid-base pair.
Strong acids will dissolve completely in water and never reach equilibrium. There is no equilibrium constant for strong acids or bases because of no equilibrium
Important strong acids
HCl, HBr, HI, HNO3, HClO4, H2SO4
Important strong bases
LiOH, NaOH, KOH, Ba(OH)2, Sr(OH)2
Because completion occurs, the acid's conjugate base must be extremely weak.
Finding the pH of strong acids is easier than finding the pH of weak acids because strong acids will dissociate completely while weak acids will only partly dissociate, so the concentration of hydrogen ions is different in the beginning versus the end.
pH = -log[H+]
[H+] = 10-pH
pOH =-log[OH-]
[OH-]= 10-pOH
pH +pOH = 14.00
pH/pOH always has 2 decimal places
The autoionization of water
Water molecules can react with each others to form H3O+ and OH-
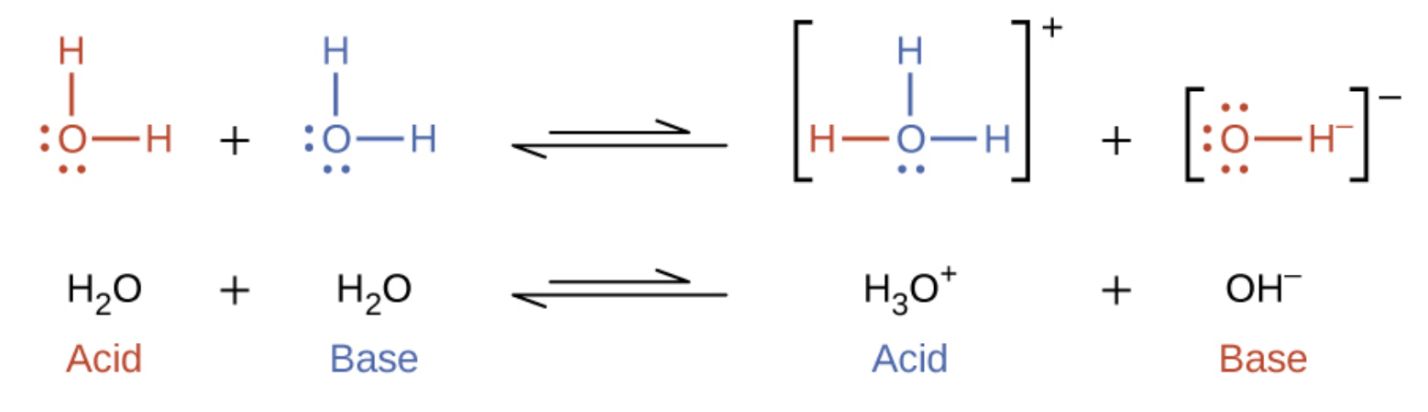
The equilibrium formed greatly favours the water molecules (reactants)
The ion product for water, Kw, is a constant value of 1.0×10-14 (no units) at SATP because bonds will form and break as the temperature changes so the Kw value changes
Kw= [H3O+] [OH-]
[H+] = [OH-] —> neutral solution
[H+] > [OH-] —> acidic
[H+] < [OH-] —> basic
Monoprotic acids have one Hydrogen atom
Diprotic acids have two Hydrogen atom
In pure water and systems at SATP, the pH is always 7.00, because the concentration of H+ ions and OH- ions are equal
The H and OH in any acid or base solution must be consistent with the ionization of water
Ka and Kb values are also consistent with the water equilibrium reaction
Kw = 1x10^-14 = Ka x Kb
pKa + pKb =14
pHs can exceed 14 and be lower than 0, but are uncommon
Weak acids
weak acids partially ionize in water to form hydrogen ions
i.e carboxylic acids
For a substance to act as a base, it must contain a lone pair in which an H+ from water will attach and form OH- and HB (B = any base)
Acids have an ionization constant, Ka, where the equilibrium concentration of the products is divided by the equilibrium concentration of the reactants (excluding water because its concentration is too large to cause any changes).
for bases, Kb
Kw = Ka x Kb
The larger the Ka, the lower the Kb; the stronger the acid, the weaker its conjugate base pair
Large Ka = strong acid
Polyprotic acids can give up more than one hydrogen in a solution
H2SO4 and H3PO4 are polyprotic
kind of acts as common ion effect
The first proton is easier to give up than the second one
the first ionization matters most for the pH
Chemical Kinetics
chemical kinetics is the study of ways to make chemical reactions go faster or slower
reaction rate is the speed at which a chemical change occurs
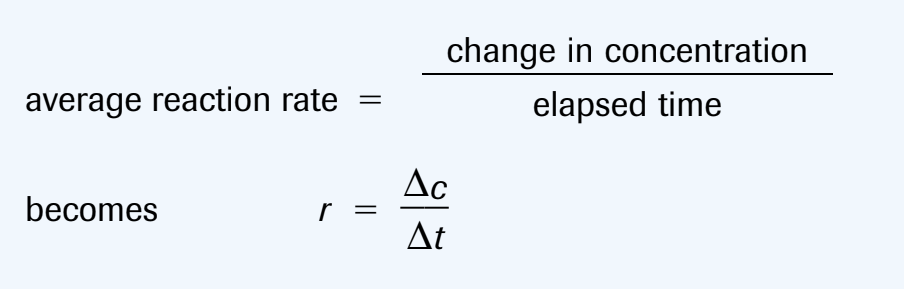
On a graph, the average rate of reaction over a time period is the absolute value of the slope of the secant
The instantaneous rate of reaction is the slope of the tangent
(-) sign indicate a rate of consumption of the reactants
(+) sign indicate a rate of production of the products
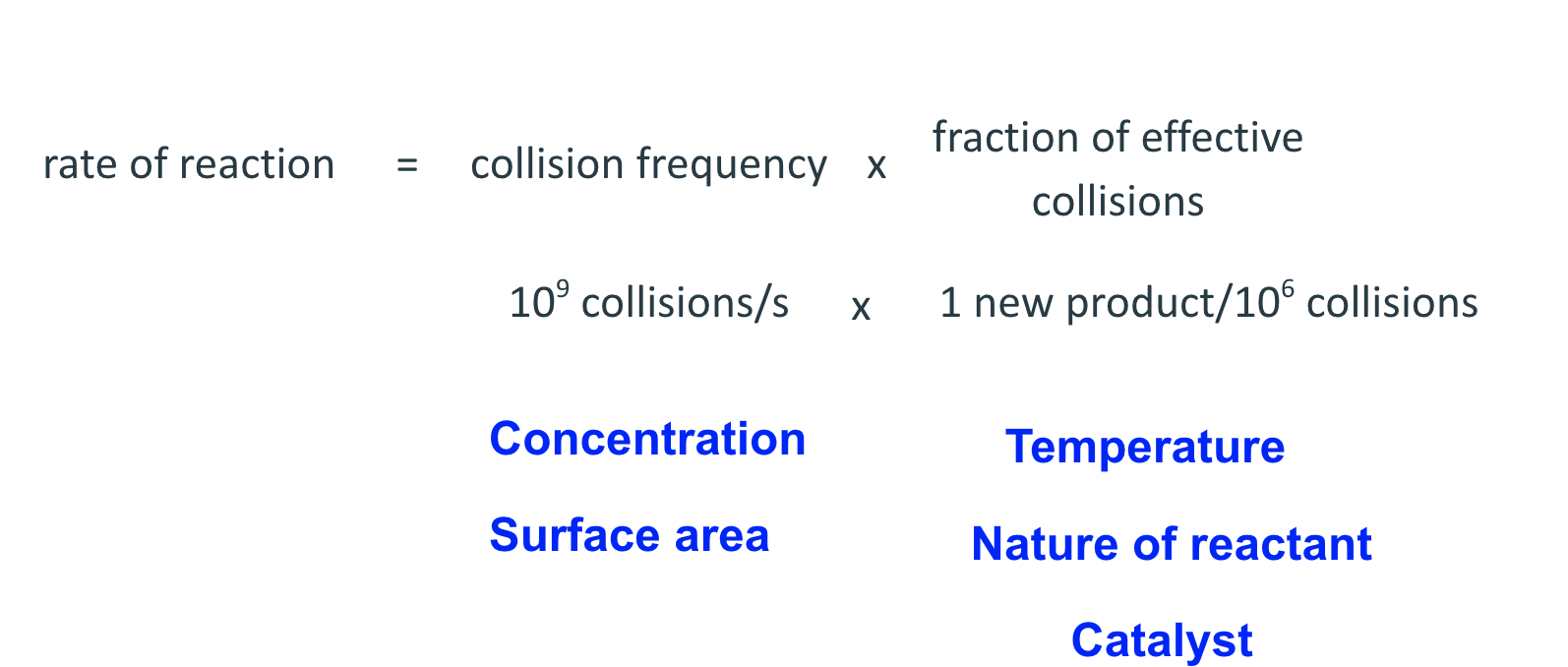
Factors that affect rates:
nature of reactants :
concentration of reactants: if the initial concentration of a reactant is increased, then the reaction rate increase
Temperature: Chemical reactions typically occur faster at higher temperatures
presence of catalyst: speeds up a reaction by providing an alternative route, requiring a lower activation energy, EA (large k value)
surface area: reaction rates increases proportionally with an increase in surface area
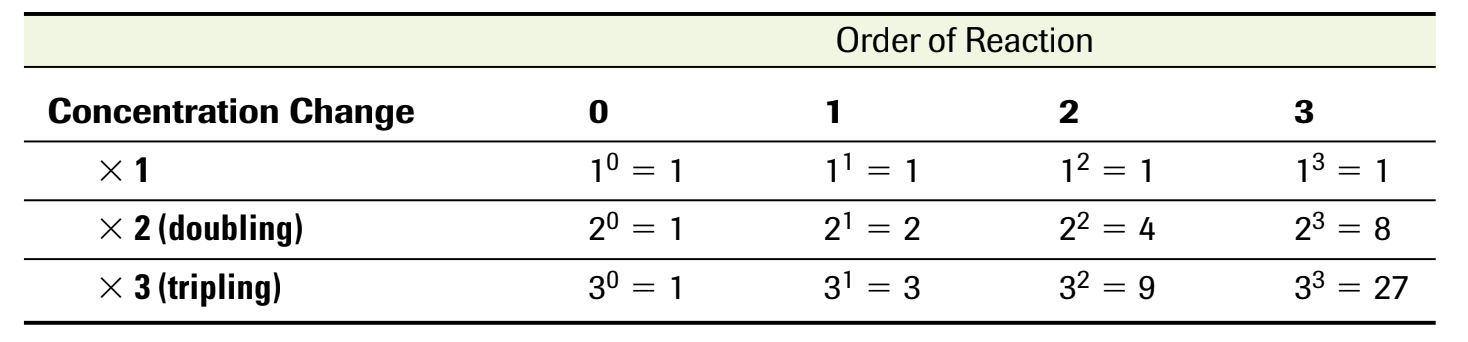
Rate is always proportional to the product of the initial concentration of the reactants where these concentration are raised to some exponent
(k is the rate constant - determined through calculations and only at specific temperatures)
m and n are exponents that describe the relationship between rate and initial concentration and can only be determined empirically.
can be all real numbers (0, fractions…)
don’t have to be equal to the coefficients in the balanced equation.
The order of reaction is the value of the exponent that describe the initial concentration dependence of a reactant
overall order of reactant is the sum of the exponents of the rate law equation
ex. r= k[NO2]1[F2]1
order with respect to NO2 is 1
order with respect to F2 is 1
overall order is 2 (1+1)
Rate Law Using Initial Concentrations
The rate law of a reaction is based on the initial concentrations of the reactants, the rate of the disappearance of products and the appearance of the products and requires experimental data. Arrhenius constant, k, is based on the activation energy for a reaction and the temperature.
Rate = k[A]^x[B]^y[C]^x
The value of the exponent is based on how the reactants affect the rate. Larger exponent means the reactant concentration changes more. To find the exponent value, find what happens when a concentration is doubled.
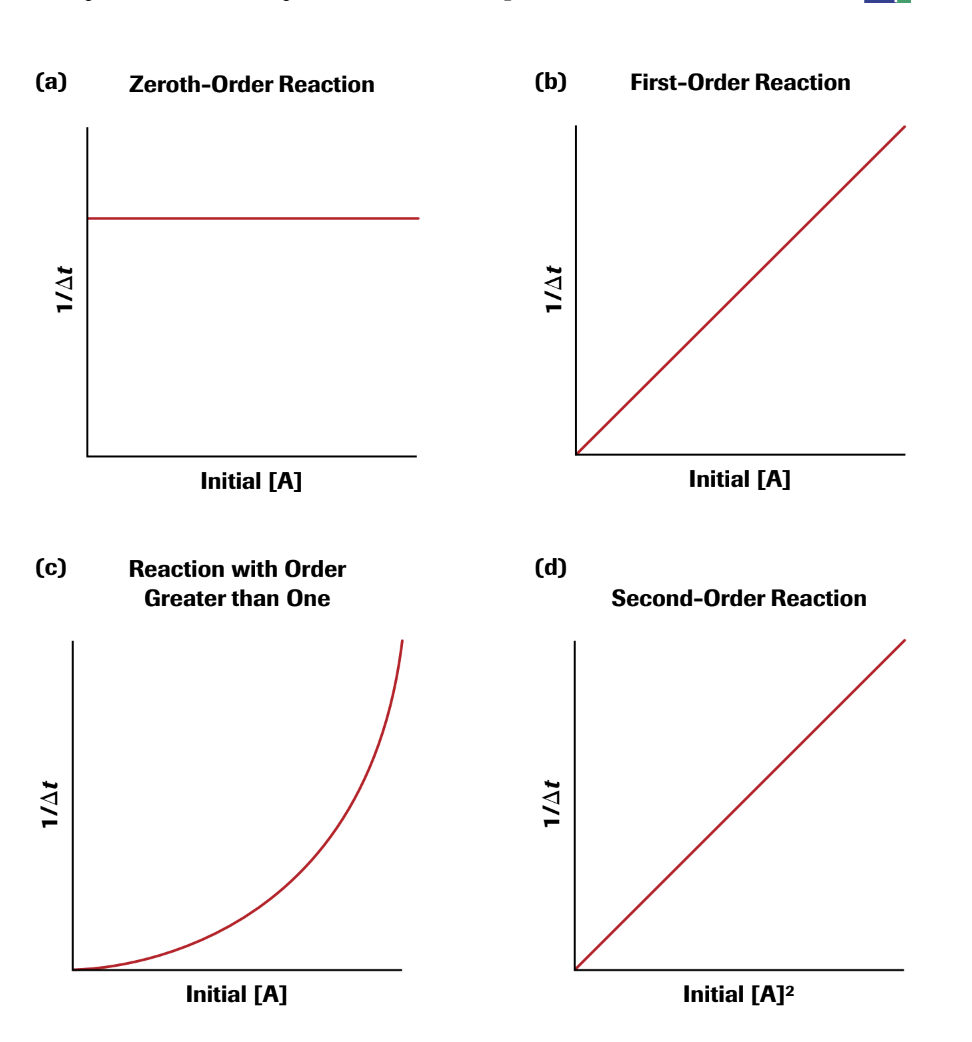
Rate law can be expressed using concentration changes over time. There are zero, first, and second order rate laws that each have the roan equation and graph.
Zero-Order Rate Laws
In zero order, the rate is always the same at a given temperature and doesn't change based on reactant concentration.
Rate = k
The graph is a straight line equal to -k and concentration vs. time.
First- Order Rate Laws
First order rate law is based on the concentration of one reactant to the first power.
Rate = k[A]
The graph is the natural log of [A] vs. Time. This creates a straight line with a slope of -k and y-interest of ln[A] at time 0.
Collision Theory
Collision theory states reactions only occur when chemicals collide with each other with sufficient energy (activation energy).
They can react more if there's a higher concentration of aqueous or gaseous substances, or a high surface area of a solid substance.
Stirring can sometimes speed up a reaction. If the mixture is heterogeneous, not all parts of a mixture is identical, then stirring will mix it more and speed it up. If the mixture is homogeneous, where the mixture is completely identical, then stirring will not help because it is already mixed and even.
Reaction rate increases with temperature because molecules are moving faster and have a higher likelihood to collide with other molecules at a sufficient speed.
Molecules will only react if they collide with the correct orientation.
Reaction Mechanisms
Some reactions occur in multiple steps rather than in one step and the overall balanced reaction is the sum. The in between steps are called elementary steps.
Example: The rate for elementary steps can be determined by setting the concentration of a substance to the power of it’s coefficient
I. A + A ⇌ X (fast) Rate=k[A]^2
II. X + B → C + Y (slow) Rate=k[X][B]
III. Y + B → D (fast) Rate=k[Y][B]
X and Y are intermediates, they are created in one step and consumed in another, so they cancel out.
If an elementary step has only one reactant, it is unimolecular. If it has two reactants (even if they are the same reactant, like in step l), it is considered bimolecular.
The slowest step is considered the rate-determining step because the speed of the reaction cannot exceed the slowest step. This step determines the rate law for the entire reaction.
Step II has an intermediate, which is not written in the reaction, and it is produced in step I, so X can be replaced with [A]^2. The rate law for step two and the overall reaction becomes Rate=k[A]^2[B].
a multi - step reaction can be written as a reaction energy profile diagram
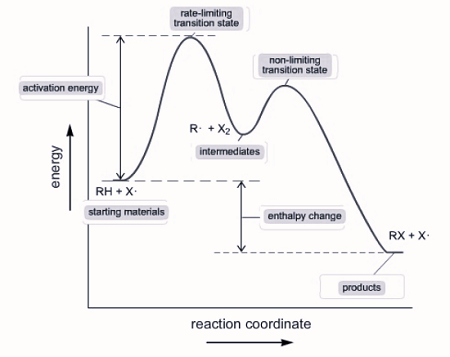
Catalysts
Catalysts are added to a reaction to speed it up without being consumed. Catalysts are present in the beginning and end of elementary steps and cancel out.
For the steps in the reaction A + B → C
I. A+ X→ Y
II. B + Y → C + X
In this reaction, x is the catalyst and Y is the intermediate