Chemistry Chapter 10 (copy)
What is the core concept of this chapter?
Chemical symbols, formulas, and equations can be used to concisely represent elements, compounds, and what happens in a chemical reaction
What are the different types of chemical formulas?
Empircal formula, Molecular Formula, and Structural Formula
Empircal Formula - identifies the elements present in terms of simplest whole # ratios
ex. CH2O
Molecular Formula - Identifies actual number of atoms in a molecular compound
ex. C6H12O6
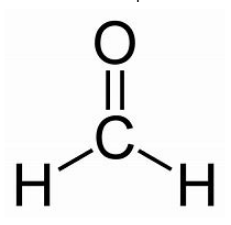
Structural Formula - Represents arracngement of atoms within a molecule; related to 3-D structures of molecules
ex. ⬇
Empiral vs. Molecular Formula?
Empircal: Ionic-lacking discrete unit, or molecule, composed of both metallic and nonmetallic elements, electronegativty difference 71.7
ex. CH20
Molecular: Covalent compounds, usually nonmetals bound to nonmetals, molecular and empirical formulas can be different
ex. C6H12O6
Molecular and Formula Mass
Formula mass - sum of all atomic masses of all atoms in chemical formula
Molecular mass - Formula mass of a molecular substance; often used for nonmolecular substances as well
Chemical Reactions
Those that give off energy are exothermic
ex. engine, fire, handwarmers, combustion, bombs
Those that take in energy are endothermic
ex. cold pack, cooking a steak, frying eggs, photosynthesis
Some reactions need an energy boos to get the reaction started, this is called activation energy
ex. match, flint, sparking
Types of Chemical Reactions
1) Synthesis reaction: combining reactants to form a new compound
ex. A + B → AB
2) Decomposition Reaction: Breaking a compound into smaller parts
ex. AB → A + B
3) Single Displacement: 1 element and 1 compound react to form a different element and a different compound
ex. A + BC → AC + B
4) Double Displacement: The components of two compounds change places. A precipitate is formed
ex. AB + CD → AD + CB
Oxidation-Reduction Reaction
Oxidation - loss of electrons
Reduction - gain of electrons
Ion Exchange Reaction
A reaction that takes place when the ions of one compound interact with the ions of another compound forming: a solid precipitate, a gas, and water
ex. AX + BY → AY + BX
Balancing Chemical Equations
Law of Conversation of Mass - Matter is neither created nor destroyed during a chemical reaction
To balance a chemical equation, you have to make sure that the atoms present at one side of a reaction are also displayed on the other side of the reaction
Chemical Equations (Word)
What is this chemical equation in word form?
CA + 2H20 → Ca(OH)2 + H2
Answer: One molecule of calcium plus two molecules of water yields one molecule of calcium hydroxide plus one molecule of hydrogen gas
What is this chemical equation in number form?
Two molecules of hydrogen gas plus one molecule of oxygen gas yields two molecules of water.
Answer: 2H2 + O2 → 2H20
Ch.10 Formula Mass
Formula Mass - The sum of the atomic masses of the elements in a chemical formula
Moles
Important concepts of the mole
Recall that the atomic weight of elements are average relative masses of the isotopes of the element. Everything is compared to Carbon-12
Carbon-12 has exactly 6.02 × 10(23) atoms. This is Avogardo’s number.
Avogardo said that equal volumes of gases contain equal number of molecules.
An amount of a substance that contains Avogardo’s number of atoms, ions, or molecules is defined as a mole of that substance