OCR Gateway Chemistry A - Particles Revision Guide
OCR Chemistry A Revision Guide
Unit 1: Particles
Particle Theory of Matter
In the Particle theory of Matter all matter is made up of small particles. This particle model is used to explain the physical characteristics of the states of matter: solid, liquid and gas.
States of Matter
At 0℃: water changes state from solid to liquid. This temperature is called the melting point.
When water is heated, the particles will move faster as energy is provided to the particles.
At 100℃: water changes state from liquid to gas. This temperature is called the boiling point.
As any matter is changing state, you will find that the temperature will remain constant during this process.
Changing state when heating, such as in evaporation or boiling, requires energy and this energy is used to overcome the forces of attraction between particles.
Changing state when cooling, such as in condensation or freezing, the particles lose energy into the environment and forms forces of attraction between particles to bring them closer together.
Particle Arrangement and Movement
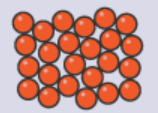
Solids:
Particles are very close to each other.
Particles have an organised and regular pattern.
Particles vibrate in one position.
Particles have low energy.

Liquids:
Particles are close to each other.
Particles are arranged randomly.
Particles slip and slide over each other.
Particles have greater energy than in solids.
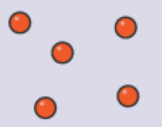
Gases:
Particles are far apart.
Particles are arranged randomly.
Particles move quickly without direction.
Particles have high energy.
Properties of States of Matter
Solids:
Solids have a fixed volume and shape.
It can’t be easily compressed because there is no extra space for the particles to occupy.
Liquids:
Liquids have a fixed volume but variable shape.
It can flow and adopts shape of any container it’s put into because particles can slip and slide over each other.
It can’t be easily compressed because particles are close together without extra space to occupy.
Gases:
Gases have no fixed shape or volume.
It can flow and can completely occupy their containers because particles move quickly and distribute across all directions.
It can be compressed because particles are far apart with extra space to occupy.
Physical and Chemical Changes
Changes in states of matter are physical changes that are easily reversible.
In chemical changes, a new substance can be formed as a result and usually this change can't be reversed easily.
Particles split up from one another and rejoin in a different arrangement.
Limitations of the Particle Model
The particle model, although useful in understanding particles, also has a drawback in its theory.
It fails to consider:
The forces of attraction between particles,
Size of particles,
The fact that there is space between the particles.
Atoms
An atom is the smallest part of an element
Atoms have a positively charged nucleus at its centre with negatively charged electrons surrounding it.
Typical size of atoms and small molecules is approximately 1 × 10-10 m.
Radius of the nucleus which is about 1-10 m is much smaller compared to that of the entire atom
Nucleus consists of protons and neutrons, and carries most of the mass in its nucleus.
Atoms contain equal numbers of protons and electrons.
Protons carry a positive charge while electrons carry a negative charge so overall charge is neutral (zero) as the charges cancel each other out.
The Atomic Model
Dalton’s model (1803)
John Dalton had the idea that all matter was made up of tiny particles known as atoms.
His model included these concepts:
Atoms are the smallest part of an element and so can’t be broken down into a simpler substance.
Atoms of a given element are identical to each other.
Atoms of different elements are different to one another.
In chemical reactions, atoms rearrange themselves to forms a different substance.
Thomson’s Model (1897)
J.J. Thomson made a discovery of the negatively charged electron and established the ‘Plum Pudding Model’ involving the even distribution of charges throughout the atom.
This model states that:
Atoms are spheres of positive charge.
Electrons are dotted around inside.
Geiger-Marsden Model (1911)
Ernest Rutherford along with Geiger and Marsden put the Plum Pudding model to test.
They found out that at the centre of the atom carries the most density called the nucleus.
They proved this by aiming alpha particles at thin gold foil.
These particles were supposed to pass right through in terms of the Plum Pudding model, however in the experiment it was found that many of them had changed direction.
Rutherford explains the experiment results:
Atoms have a central, positively charged nucleus consisting of the majority of the mass.
Electrons surround the nucleus as if in orbit.
Bohr’s Model (1913)
Niehl Bohr had foreseen that electrons occupy energy levels or orbitals.
The atomic model that exists at present times has taken centuries to reach, it has been done through the help of experimental evidence.
KEYWORDS:
Mass number - total number of protons and neutrons in an atom.
Atomic number - number of protons in an atom.
Ion - formed as a result of an element gaining or losing electrons.
Isotopes - atoms of the same element that have the same atomic number but different mass number due to a difference of neutrons.
Subatomic Particles
Proton:
Charge: +1
Relative mass: 1
Neutron:
Charge: 0
Relative mass: 1
Electron:
Charge: -1
Relative mass: 0.0005 (practically zero)
Calculating Numbers of Subatomic Particles

When calculating the numbers of subatomic particles in an atom:
Number of Protons = Atomic number
Number of Electrons = Atomic number
Number of Neutrons = (Mass number) - (Atomic number)
In this example with chlorine:
Protons = 17
Electrons = 17
Neutrons = 35-17 = 18