phys cba1 notes!
kinetic particle model of matter
matter
matter is made up of tiny particles in continuous, random motion
note: singular air particles move in one direction. if asked to draw the motion of ONE air particle, it can be drawn bouncing off surfaces. however, when asked to draw the motion of an air particle among a large number of air molecules, it would have to hit other air particles on the way → the path will appear to be zigzagged.
brownian motion
refers to the random motion of tiny particles suspended in a fluid (e.g. smoke in air or pollen in water)
in experiment, bright specks of light (which are the smoke particles) were observed to be moving about in a constant, random motion.
this is because the smaller air particles are also in constant, random motion. the air particles collide unevenly with the larger smoke particles (from all directions) and exert a resultant force on the smoke particles. the smoke particles move in the direction of the resultant force, as in Newton’s 2nd Law of Motion.
only particles that are relatively big enough to be seen, and that are light enough to be moved by air particles can be used in above experiment.
kinetic model of matter
property | solid | liquid | gas |
diagram representation of particles |  | 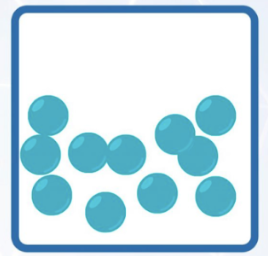 |  |
arrangement | orderly arrangement, packed closely together | not in orderly arrangement. packed closely together | not in orderly arrangement, far apart from each other |
motion | vibrate about a fixed position (low KE) | free to move around within vessel and slide over each other | free to move at high speeds (high KE) |
force of attraction | very strong attractive forces | strong attractive forces | weak attractive forces |
properties in relation to states
property | solid | liquid | gas |
compressibility (explain using packing/distance between molecules) | incompressible | incompressible | compressible |
shape and volume (explain using forces and motion of particles) | fixed shape and volume | fixed volume, takes shape of vessel | no fixed shape or volume |
its generally true that the volume of substance in the solid state is equal to the volume of substance in the liquid state.
temperature of matter and kinetic energy
as the temperature of energy increases, the kinetic energy it possesses will increase as well.
note: during change of state, energy is related to potential energy, average kinetic energy stays the same during state change. hence:
when a piece of ice melts, intermolecular forces between molecules become weaker while the molecules move faster.
when a substance is heated without any change in state, the intermolecular forces stay the same while the molecules move faster. (distance stays the same)
when spacing between particles increases, its potential energy increases.
gases
equations: (relationship: one causes the other)
P1/T1 = P2/T2
V1/T1 = V2/T2
P1V1 = P2V2
P1V1 / T1 = P2V2 / T2
PV = nRT: this equation is not tested, but can be used to remember the relationships between different variables. main variables to be tested are pressure, temp and volume only.
P → pressure
V → volume
n → no. of moles
R → universal gas constant
T → temperature (Kelvin)
pressure vs temperature | volume vs temperature | pressure vs volume |
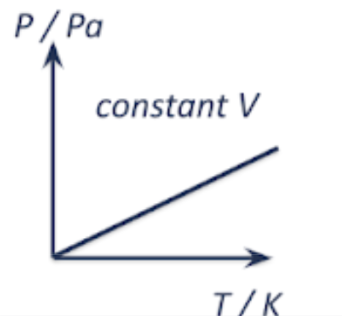 as temp increases, the average KE of gas molecules increases, and the molecules move faster and collide with the walls of the container more frequently and with greater force.
| 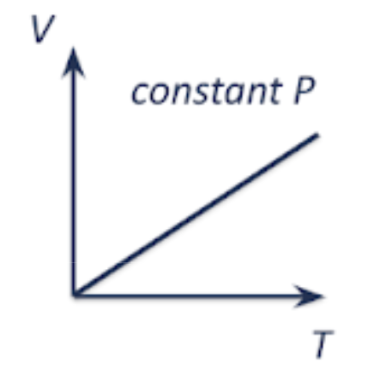 as temp increases, the average KE of gas molecules increases, and the molecules move faster and collide with the walls of the container more frequently and with greater force. if pressure is kept constant, the volume of the gas needs to increase. | 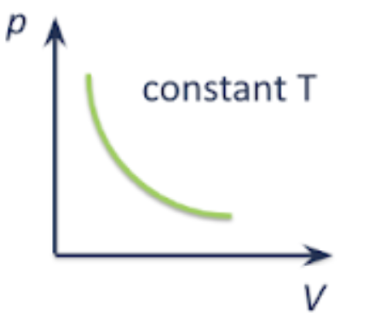 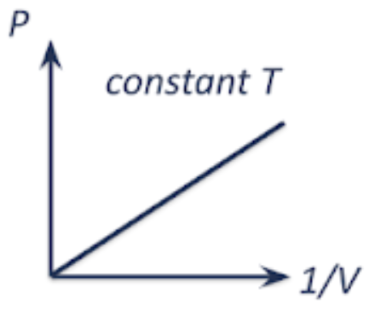 as volume increases, the number of gas molecules per unit volume decreases. there are less frequent collisions with the wall of the container, resulting in a lower force per unit area being exerted. hence, pressure decreases. |
faqs and notes
common questions: |
State and explain the pressure changes inside the balloon after the vacuum pump is switched on. When some air is removed, the pressure in the bell jar decreases and becomes lower than atmospheric pressure. Difference in pressure The balloon increases in size because the external pressure decreases. Equilibrium There are now fewer collisions with the walls of the balloon as its volume has increased while the no. of particles is the same. Explain (Volume vs Pressure) Hence, the pressure in the balloon decreases until the pressure in the balloon is equal to that outside it. |
Explain why frequency of collisions remains the same. Since the volume of the gas is greater, there are less molecules per unit volume and they will a longer time to reach the walls of the cylinder. Hence, there is a decrease in the number of collisions per unit time, resulting in the pressure remaining at the same value. |
notes:
volume is directly related to distance between particles. (as volume increases, distance between particles also increases ALWAYS → given the no. of particles remains the same)
frequency of collisions and speed of particles are both INDIVIDUALLY directly related to pressure:
if pressure remains unchanged at a higher temp, we would expect some other variable (volume and temp) to have changed such that the frequency of collisions decreases.
cannot say gas molecules gained TE: TE is supplied to the gas and is converted to KE of the molecules.
thermal properties of matter
temperature
measures the concentration of thermal energy
temperature is a property of a material, and hence depends on the material, whereas TE is a form of energy existing on its own.
celsius and kelvin scale
Tk = Tc + 273.15
pure melting ice (ice point) | pure steam (Steam point) | zero kelvin (absolute zero) | |
kelvin | 273.15 | 373.15 | 0 |
celcius | 0 | 100 | -273.15 |
at zero kelvin, molecules have stopped moving and the pressure would be 0.
heat
a measure of how much thermal energy is transferred from one body to another. transfer of energy from one body to another is a result of temperature differences between them.
thermal equilibrium
net heat flows from hotter to colder object, until both are of the same temperature. both objects are said to be in thermal equilibrium when there is no net heat flow between them. (there is still heat flow, just no NET heat flow.
zeroth law of thermal dynamics (NOT TESTED): when a body A is in thermal equilibrium with another body B, and also separately in thermal equilibrium with a body C, then both body B and C will also be in thermal equilibrium with each other.
internal energy
internal energy of a body is the total kinetic energy due to the motion of the molecules and potential energy due to the intermolecular forces in the body (IE = KE + PE)
KE (change in temp) | PE (change in state) |
KE increases as temp increases: the higher the temperature, the more vigorously the particles vibrate/move | PE increases during melting/boiling: particles move further apart. this is due to energy being absorbed to weaken the bonds of the particles. |
KE decreases as temp decreases: the lower the temperature, the less vigorously the particles vibrate/move | PE decreases during solidification /condensation: particles move closer together. |
regardless of how the temperature is varying, the internal energy of the system simply increases linearly with energy input. | |
heat capacity
heat capacity of a body is the quantity of thermal energy needed to cause its temperature to change by 1ºC or 1K.
unit: J oC-1 or J K-1 (if value given by the question has this unit, do not use mass in calculations)
heat capacity depends on substances the object is made up of and mass of substances in the body.
formula: Q = c Δθ
c → heat capacity (J oC-1 or J K-1)
specific heat capacity: the quantity of thermal energy needed to change the temperature of 1kg of substance by 1ºC or 1K. (divided by the mass)
formula: Q = mc Δθ
Q → thermal capacity/energy of object (J)
c → specific heat capacity (J kg-1 oC-1 or J kg-1 K-1)
Δθ → change in temperature (ºC or K)
m → mass
phase change (change of state)
no net change of temperature during phase change → this is a concept called latent heat
latent heat (L) → amount of thermal energy absorbed/released during a change of state without a change in temperature (J)
specific latent heat → energy needed to change the state of m kg of a substance without a change in temperature (J kg-1)
formula: L = mlf or mlv (write Q = ml)
L → latent heat
m → mass
lf → specific latent heat of fusion
lv → specific latent heat of fusion
Heating Curve - Melting  |
latent heat of fusion: the amount of thermal energy required to change a substance from a solid to a liquid (or vice versa) without a change in temperature. |
Heating Curve - Boiling 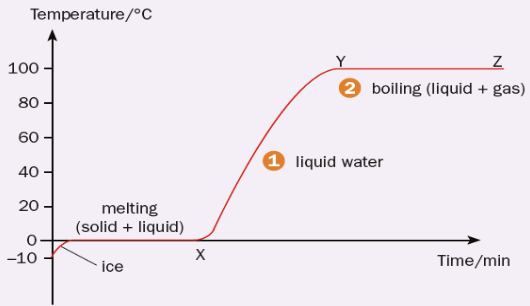 |
latent heat of vaporisation: The amount of thermal energy required to change a substance from a liquid to gas (or vice versa) without a change in temperature. |
sublimation → solid to gas
deposition → gas to solid
boiling vs. evaporation
boiling | evaporation |
boiling is the change of state from liquid to gas, without a change in temperature. the temperature in which liquid boils is called boiling point. | evaporation is the change of state of a liquid into gas, at any temperature. evaporation causes cooling. |
occurs at fixed temperature | occurs at any temperature |
occurs throughout the liquid | occurs at surface of the liquid |
occurs quickly | occurs slowly |
bubbles are formed in the liquid | no bubbles formed in the liquid |
thermal energy is supplied by an energy source | thermal energy is supplied by the surroundings |
evaporation
causes cooling: molecules that are evaporated gain TE from surroundings, and escape as vapour, carrying thermal energy away from the liquid
factors affecting rate of evaporation:
temperature: higher temperature, higher rate of evaporation
surface area: greater surface area, higher rate of evaporation
humidity: higher humidity, lower rate of evaporation
pressure: higher pressure, lower rate of evaporation
cooling curve 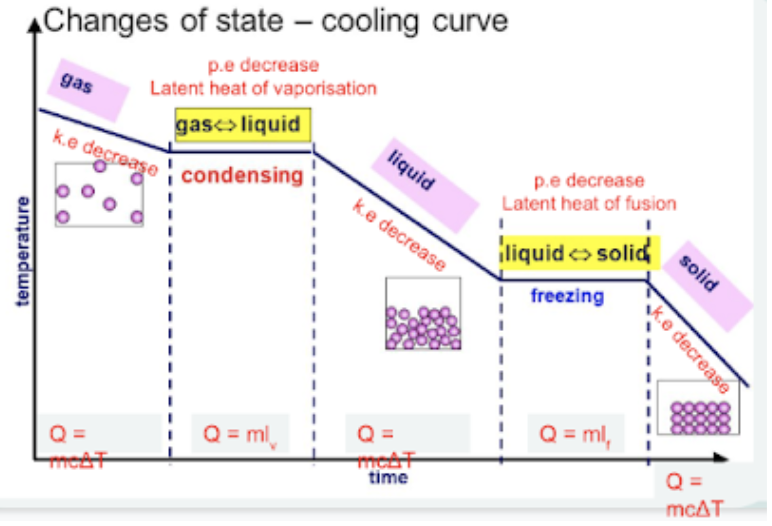 |
heating curve 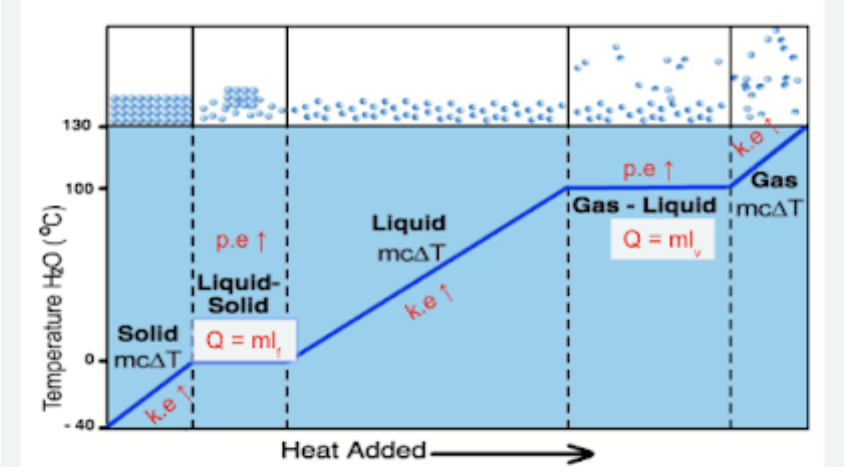 |
faqs and notes
common questions: |
A 0.500 kg block of metal with an initial temperature of 30.0oC is dropped into a container holding 1.12 kg of water at 20.0oC . If the final temperature of the block-water system is 20.4oC, what is the specific heat capacity of the metal? 0.500 x (30 - 20.4) x C = 1.12 x (20.4 - 20.0) x 4186 |
Which will cause a more severe injury? Scalding by boiling water at 100oC or steam at 100oC? Steam will cause a more severe injury as it has more thermal energy since more energy was transferred to steam to overcome the strong FOA in the phase change. |
To bring a fever down, a towel soaked with water placed on the forehead is often used. Specific Heat Capacity Water has a high specific heat capacity. Thermal Energy Explanation Hence, it absorbs a large amount of thermal energy for a corresponding small temperature rise. Link back to use Thus it brings down the temperature of a body with small increase in its temperature and is a very useful cooling agent. |
Why is the time taken for the water to boil longer in real life? Some of the thermal energy supplied by the bunsen burner is lost to the surroundings. |
Why is latent heat of vaporisation higher than latent heat of fusion? More work needs to be done to overcome the atmospheric pressure and the intermolecular forces. OR (use evidence from graph) The time taken for the wax to vaporize is greater than that taken for it to melt. |
notes:
look out for “specific heat capacity” and “heat capacity”. when explaining why an object is better for a certain purpose, use “specific heat capacity” → you are comparing the material itself directly
“per degree celsius” and “per kelvin” are the same
working:

general notes:
the length of any arrows should be drawn representative of the relative magnitude of the forces it represents
can use the answer that has been rounded off in part (a) to solve part (b)
when asked to state a quantity that needs to be measured in order to determine something, state it specifically, as if in a lab practical (e.g mass OF the block)
standard form: not required unless asked, no rule of thumb
 Knowt
Knowt