Regents Chemistry Ultimate Guide
Topic 1: Atomic Structure and Matter
Dalton’s Theory - Atoms are indivisible, atoms of an element are identical, compounds are formed from elements, atoms of different elements are different
Thomson’s Theory - Discovered the electron using the Cathode Ray Tube, electron is negative (-) charge and has very little mass
Rutherford’s Theory - Discovered the nucleus through the Gold Foil Experiment. Alpha particles passed through and some were deflected through a sheet of gold, which proves that the nucleus is small, dense, and positively charged. Atom is mostly empty space otherwise
Bohr Model - Electrons exist in shells around the nucleus. Each energy level has it’s own amount of energy.
Modern (Wave Mechanical) Model - Orbitals are regions of most probable electron location. An atom consists of a small, positive nucleus surrounded by a cloud of negative electrons.
Average Atomic Mass - Weighted average of all naturally occurring isotopes of an element:
(mass * percentage) / 100 + (mass * percentage) / 100.. repeated for as many isotopes
Atomic Spectra - When electrons move from low to high energy they absorb energy, when electrons move from high to low energy levels they release energy
Electron Configuration
Ground State (lowest energy): 2-8-18-32
Excited State (higher energy): when electrons move around shells and don’t follow the order above. THE NUMBER OF ELECTRONS DO NOT CHANGE.
Matter
Elements: Cannot be broken down by chemical means (on the periodic table)
Compounds: Composed of 2 or more elements chemically combined; can be broken down into elements.
Both of these are pure substances.
Mixtures
Combination of 2 or more pure substances than can be physically separated
Homogenous: no distinguishable difference in appearance/properties
Heterogenous: difference in appearance and properties
All solutions (aqueous) are homogenous mixtures.
Particles
Protons are positive (in nucleus)
Neutrons are neutral (in nucleus)
Electrons are negative (outside nucleus)
Atom - A neutral particle with no charge
Ions - An atom that has gained or lost electrons and now has a charge
Isotopes - An atom with different number of neutrons but same number of protons
Nuclear Charge - Charge of nucleus, only protons = atomic number
Mass Number - (top number) Number of Protons + Neutrons
Atomic Number - (bottom number) Number of protons
Number of neutrons = Mass # - Atomic #
Number of protons = # electrons in a neutral atom
Physical Separation Methods
Distillation: separation of components by differences in boiling points
Filtration: Separating a solid from a liquid in a heterogenous mixture
Chromatography: separation of components by polarities
Evaporation/Boiling: separating a homogenous solution from it’s solute
Topic 2: Nomenclature and Formula Writing
Diatomic - Occur in nature this way, not combined in a compound: Br2, I2, Cl2, H2, O2, F2
Formulas - Show qualitive info (what it is) and quantitative info (how many there are)
Molecular - How many atoms are actually there
Empirical - A simplified molecular formula
Polyatomic Ions - Listed in Table E, Do NOT break up, contains covalent bonds and forms ionic bonds
Writing Formulas
Metals (positive ions) are always written FIRST, Non-metals are are written LAST
Criss-Cross the charges of ions and make them into subscripts
Mg+2 + N-3 —> Mg3N2
Roman Numerals
Written when the metal has more than one charge (transition metals). Roman numerals state the charge of the metal. DO NOT USE if the metal has only 1 charge
Fe+2 + O-2 —> Iron (II) Oxide
Fe+3 + O-2 —> Iron (III) Oxide
Endothermic Reactions - Energy is absorbed; energy (heat) is written on the left (reactants).
ΔH = (+)
Exothermic Reactions - Energy is released; energy (heat) is written on the right side (products).
ΔH = (-)
Types of Chemical Reactions
Synthesis: A + B —> AB
Decomposition: AB —> A + B
Single Replacement: A + BC —> AB + C (element + compound —> compound + element)
Double Replacement: AB + CD —> AD + CB (compound + compound —> compound + compound)
Combustion: Hydrocarbon + O2 —> CO2 + H2O
All Single Replacement reactions are Redox Reactions
All Neutralization Reactions are Double Replacement Reactions
Law of Conservation of Mass - Mass of all reactants must Equal Mass of Products
Balancing Equations
Place coefficients in front of compounds or elements. Do NOT change subscripts. Coefficient distributes into the entire compound for each atom. Balance atoms on each side of equation.
4Fe + 3O2 —> 2Fe2O3
4 Fe on each side
6 O on each side
Topic 3: Mathematics of Chemistry
Gram Formula Mass (GFM), Formula Mass, Molecular Weight, Molecular Mass - the sum of the atomic masses of each element within the compound
Percent Composition (On Table T) - (mass of part / mass of whole compound) * 100
Mole Calculations (On Table T)
Moles = Mass / GFM
Mass = (Moles)*(GFM)
Stoichiometry / Ratios
The coefficients in a balanced equation represent moles. Find the ratio between two compounds: this is a molar ratio.
CH4 + 2O2 —> CO2 + 2H2O
CH4 and H2O are in a 1:2 ratio
3 moles of CH4 will produce 6 moles of H2O
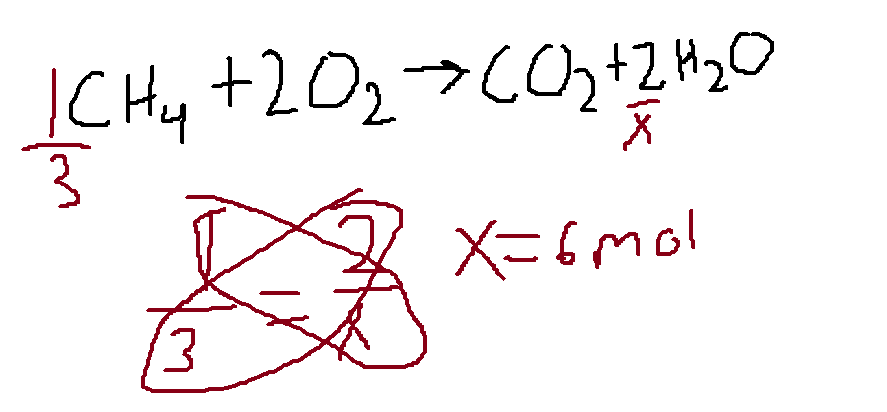
Molecular Formulas from Empirical Formulas
(Gram Formula Mass) / (Weight of Empirical Formula)
Multiple this ratio by the empirical formula to get the molecular formula
Example:
Empirical Formula: CH2 and GFM = 28 g/mol
C 1×12 = 12
H 2×1 = 2
12 + 2 + 14 g/mol
(28) / (14) = 2
2(CH2) = C2H4
Topic 4: Physical Behavior of Matter
Phases of Matter
Solid: definite shape and volume, rigid, fixed patterns
Liquids: indefinite, definite volume
Gases: indefinite shape and volume, fills container completely
Heating and Cooling Curves
Lines that are increasing (or decreasing) in temperature
Kinetic Energy is changing
Lines that are flat
Kinetic Energy is remaining the same, but Potential Energy is changing
Freezing: liquid to a solid, requires heat of fusion (334 J)
Melting: liquid to a solid, requires heat of fusion (334 J)
Condensation: gas to a liquid, requires heat of vaporization (2260 J)
Boiling/Vaporization: liquid to a gas, requires heat of vaporization (2260 J)
Sublimation: solid to a gas
Deposition: gas to a solid
Temperature is AVERAGE KINETIC ENERGY
Temperature is not affected by amount, highest temperature = highest average kinetic energy
Heat of Fusion: amount of heat needed to melt/freeze
Heat of Vaporization: amount of heat needed to vaporize/condense
Gas Law Relationships
PV / T = PV / T
(P-T-V Chart)
If something is constant or not given, remove it from the equation
Graphs:
Pressure and Volume = Inverse
Pressure and Temperature = Direct
Volume and Temperature = Direct
Ideal vs Real Gases
Volume is negligible in ideal gases
Ideal gases have no attraction
Ideal gases may not transfer energy between each other
Particles move in random, straight-line motion
Gases are more ideal at Higher Temperature and Lower Pressure (HiTLoP - high temp, low pressure)
Avogadro’s Law
Two gases that occupy the same volume have the same temperature, same pressure, and same number of molecules
The masses of the gases may be different based on their identity and GFM
Topic 5: The Periodic Table
Mendeleev - organized elements by atomic mass
Modern Periodic Law - elements are arranged by atomic number
Organization
Groups - Columns (18)
Periods - Rows (7)
Group 1 = Alkali Metals
Group 2 = Alkaline Earth Metals
Groups 3-12 = Transition Metals
Group 17 = Halogens
Group 18 = Nobel Gases
Metals (left side of table, most elements)
Non-Metals (right side of table, and hydrogen)
Metalloids (touching the diagonal except Al and Po)
Liquids = Hg, Br
Gases = H2, O2, N2 F2, Cl2, He, Ne, Ar, Rn, Xe
The rest are solid
Properties
Metals: Malleable, ductile, conductive, luster, low ionization energy and electronegativity, lose electrons and form positive smaller ions
Non-Metals: Brittle, non-conductive, dull, high ionization energy and electronegativity, gain electrons and form negative larger ions
Metalloids: properties of both metals and non-metals
Nobel Gases - unreactive due to full valence shell (8 electrons)
Allotropes - two or more forms of the same element in the same phase, ex. O2 and O3
Different Molecular Structures = Different Properties
Trends
Atomic Radius: size of the atom
Across a period: radius DECREASES
Down a group: radius INCREASES
Ionization Energy: amount of energy needed to remove electrons in an atom
Across a period: IE INCREASES
Down a group: IE DECREASES
Electronegativity: an atoms attraction for electrons
Across a period: EN INCREASES
Down a group: EN DECREASES
Groups
Groups are organized by the number of valence electrons each atom possesses
Group 1 has 1 valence electron, Group 13 has 3, Group 15 has 5, etc..
Elements within the same group have similar chemical properties due to the same number of valence electrons.
Periods
Organized by number of electron shells, periods do NOT have similar properties
Topic 6: Bond Polarity
Bond Energy
Breaking Bonds —> Absorbs Energy
Forming Bonds —> Releases Energy
B.A.R.F
Break Absorb, Release Form
Types of Bonds
Covalent: between 2 or more non-metals (sharing of electrons)
Ionic: between a metal and a non-metal (transfer of electrons)
Metallic: just a metal (sea of mobile electrons)
Types of Solids/Properties
Covalent/Molecular: Low melting point/boiling point, soft, does NOT conduct electricity
Ionic: High melting point/boiling point, hard, conduct electricity only in AQUEOUS or LIQUID phase, NOT solid
Metallic: High melting point/boiling point, hard, conduct in solid and liquid phases
Lewis Dot Structures
Show only valence electrons of atom, the most dots an atom can have is 8 (Noble Gas Configuration)
Covalent Structures: between 2 non-metals atoms
Each line = 2 electrons (a pair of electrons)
Ionic Structures: between metal and non-metal
Have brackets, not lines
Both ions must have charges present
Metals have zero dots and a positive charge
Non-Metals have eight dots in a bracket and a negative charge
Ions form to achieve Noble Gas Configuration
All diatomic elements are Non-Polar with Non-Polar bonds
H2O and NH3 are Polar because of lone pairs
CCl4, CO2, and CH4 are Non-Polar with Polar Bonds
Bond Polarity
S.N.A.P
Symmetrical = Non-Polar, Asymmetrical = Polar
Polar: unequal distribution of charge, one side has electrons more often (the more electronegative side)
Polar Bond: 2 different elements
Non-Polar: equal distribution of charge, electrons are shared equally among all sides of the molecule
Non-Polar Bond: same element on both sides of the bond
Intermolecular Forces
Between covalent compounds ONLY
Non-Polar molecule = London Dispersion Forces (weakest)
Polar Molecules = Dipole-Dipole Forces
H2O, NH3, HF (H- with F, O, or N) = Hydrogen Bonding (strongest)
Intermolecular forces determine melting point/boiling point
Weaker forces = lower melting/boiling point
Strongest forces = higher melting/boiling point
Topic 7: Solutions
Formulas (Table T)
Moles = mass / GFM
Molarity = moles / liters
Parts Per Million = (part/whole) * 1,000,000 or 1×106
Solute: substance that is being dissolved
Solvent: substance that is dissolved into (usually water)
Solution: solvent + solute
Solubility Factors
Temperature: increase temperature = increase solubility (NOT gases)
Gases: Increase temperature = decrease solubility
Pressure (only gases): increase pressure = increase solubility
Nature of Solvents & Solutes: “like dissolves like”
Polar (water) dissolves Polar
Non-Polar dissolves Non-Polar
Ionic substances dissolve in Polar solvents
Table G (Solubility Curve)
Read in 100g of water, alter as necessary
If 200g of water = double amount from table
If 50g of water = half the amount from the table
A solubility reading ABOVE the line = SUPERSATURATED
A solubility reading ON the line = SATURATED
A solubility reading BELOW the line = UNSATURATED
When given 2 values to read, always subtract the values
If solute remain on bottom of beaker: Saturated solution (at equilibrium)
If solute dissolved: Unsaturated solution
If solute causes precipitation of more particles: Supersaturated Solution
Colligative Properties
Adding a solute to a solvent LOWERS the freezing point and RAISES the boiling point
The more ions it breaks into, or the higher concentration, the more effect it has on the freezing point and boiling point
Covalent compounds do NOT break up
Vapor Pressure (Table H)
Shows vapor pressure of substances at given temperatures
Increase temperature = Increase vapor pressure (for any substance, direct relationship)
Boiling: when atmospheric pressure = vapor pressure (can be any temperature)
Lower VP: Stronger Intermolecular Forces and Higher Boiling Point (Ethanoic Acid)
Higher VP: Weaker Intermolecular Forces and Lower Boiling Point (Propanone)
Topic 8: Kinetics and Equilibrium
Collision Theory
Collisions between particles must have proper energy and proper orientation
which make effective collisions occur
Factors that Affect Rate of a Reaction
Concentration: higher concentration = faster rate and more effective collisions
Temperature: higher temperature = more collisions and faster rate
Surface Area: more surface area = more area for more effect collisions
Pressure (only gases): higher pressure = more collisions
Nature of Reactants: covalent reacts slower; ionic reacts faster
Catalyst: lowers activation energy by providing an alternate pathway
Potential Energy Diagrams
Anything that starts from the bottom (zero) are potential energies
Reactants: left side
Products: right side
Forward Activation Energy: left side
Reverse Activation Energy: right side
Heat of Reaction (ΔH) = PE Products - PE Reactants
Equilibrium
Rates are Equal and Concentration is Constant
Phase Equilibrium: same compound or element but in different phases
Solution Equilibrium: same compound or element but in one phase is in solution
Chemical Equilibrium: a state in which the rate of the forward reaction equals the rate of the backward reaction
On a graph: flat lines mean concentration is remaining constant
LeChatlier’s Principle
Increase Concentration or Temperature: shift to opposite side of what you’re increasing
Decrease Concentration or Temperature: shift to same side of what you’re decreasing
Increase Pressure: shift to side with less moles of gas
Decrease Pressure: shift to side with more moles of gas
Side shifting toward: concentration increases
Side shifting away from: concentration decreases
Forward Reaction: left to right
Reverse Reaction: right to left
Catalysts: speed up reactions, but do not increase concentration
Entropy (disorder)
Solids have the least entropy, gases have the most entropy
The side with more moles has entropy
Increase Temperature = Increase Entropy
Nature prefers lower energy and higher entropy
Topic 9: Redox and Electrochemistry
Oxidation
Electrons appear on product (right) side
Oxidation number INCREASES
Electrons DECREASE
Reduction
Electrons appear on reactant (left) side
Oxidation number DECREASES
Electrons INCREASE
Oxidation Numbers
(follow rules in order of priority)
All uncombined elements (not in a compound) have an oxidation number of zero
Ions in a compound have an oxidation numbers equal to their charges
Group 1 metals = +1, Group 2 metals = +2, Fluorine = -1, (Cl, Br, I are too unless with something more electronegative)
H = +1 (unless ONLY with a metal then its -1)
O = -2 (with Fluorine O = +2, in peroxide O2-2 = -1)
Sum of all oxidation numbers in compounds = 0 or stated charge if a polyatomic ion
Redox Reactions
Single Replacement, Synthesis and Decomposition are Redox Reactions
Any reaction where an element changes oxidation numbers
Look for single elements somewhere
Double Replacements are NEVER redox
Table J (Activity Series)
Single Element must be higher than element in compound to replace (occur spontaneously)
Higher elements are oxidation (anode), lower elements are reduction (anode)
Mnemonics
O.I.L.R.I.G
OIL - Oxidation is Loss (of electrons)
RIG - Reduction is Gain (of electrons)
An Ox - Anode Oxidation
Red Cat - Reduction Cathode
VAN - Voltaic cell Anode is Negative
APE - Anode is Positive in Electrolytic cell
Voltaic (Electrochemical) Cell
Spontaneous, require no external energy
Chemical Energy —> Electric Energy
Salt Bridge: migration of ions
Wire: migration of electrons
Electrons flow from Anode to Cathode
(Away from Anode)
Anode = negative (-)
Half Reaction
Show only the Oxidation or Reduction
MUST show charges
Combined together (with balanced and canceled electrons) = full redox
The element not changing oxidation number from side to side = spectator (cross it out)
Electrolytic Cell
Non-Spontaneous, requires a battery
Electrical Energy —> Chemical Energy
NO Salt Bridge
Electrons flow from Anode to Cathode
(Away from Anode)
Used for Electroplating
Positive Ions flow to negative cathode
Negative Ions flow to positive anode
Agents
Oxidizing Agent: causes oxidation, so it’s reduced
Reducing Agent: causes reduction, so it oxidized
Topic 10: Acids, Bases and Salts
Table K - Common Acids
Table L - Common Bases
Table M - Indicators
Acids: Begin with H
Bases: Metal + OH
Properties
Acids: taste sour, turns litmus red, pH less than 7
Bases: feel slippery, taste bitter, turns litmus blue, pH greater than 7
Both: conduct electricity, react with each other to form salt and water
Electrolytes: acids, bases and ionic salts (substances that conduct electricity)
Acid/Base Theory
Arrhenius Theory
Acid: substance that donate H+ as it’s only positive ion
Base: substance that donates OH- as it’s only negative ion
Bronsted-Lowry Theory (Alternate Theory)
Acid: substance that donates an H+ ion
Base: substance that accept an H+ ion
(form conjugate pairs)
Acid has 1 more H+
Base has 1 less H+
Increase [H+], Decrease [OH-] = more acidic, lower pH
Decrease [H+], Increase [OH-] = more basic, higher pH
Neutralization Reaction
acid + base —> salt + water
(double replacement reaction)
Titration
Adding measured volumes of a known acid/base to an unknown concentration of acid/base to reach neutralization
MAVA = MBVB
(M = molarity, V = volume)
Don’t forget to count the H’s and OH’s
pH
Scale of 1-14
pH 1-6 = Acid
pH 7 = Neutral
pH 8-14 = Base
Each power of 10 for concentration = 1 pH unit
ex:
pH = 7 hundredfold increase hydronium —> pH 5 (102)
Indicators
Change color through specific pH ranges (Table M)
Topic 11: Organic Chemistry
All organic compounds contain carbon because of it’s ability to bond to itself multiple times
Saturated Compounds - all single C-C bonds (alkanes)
Unsaturated Compounds - at least 1 or double C-C bonds (alkenes and alkynes)
Naming Compounds
Name longest carbon chains with Table P and Table Q
Use number to identify which carbona group is hanging off o
A carbon group can NOT be put on the first or last carbons; it will just extend the longest chain
Functional Groups (Table R)
Look for “O” or “N” and what’s around it
Distinguish between CO, COO, COOH and OH
Circle CxHy groups
Isomers
Same molecular formula (same GFM), different structural formula
Different Structures = Different Properties = Different Functional Groups
Organic Reactions
Addition: with UNSATURATED hydrocarbons (alkene and alkyne)
Substitution: with SATURATED hydrocarbons (alkane)
Combustion: always produces CO2 AND H2O
Esterification: Alcohol + Organic Acid —> Ester + Water
Fermentation: Sugar (C6H12O6) —> Alcohol + Carbon Dioxide
Saponification: Ester + Base —> Acid + Alcohol (process of making soap)
Polymerization: Repeating units —> Polymers (involves smaller molecules joining to make one big molecule)
Cracking: long chain hydrocarbon —> small chain hydrocarbon
Topic 12: Nuclear Chemistry
Table N - Half Lives
Table O - Decay Symbols
Particles
Alpha: heaviest particle (greatest mass), positive charge, attracted to negative plate (low energy), least penetrating power
Beta: no mass, negative charge, attracted to positive plate
Gamma: no mass, no charge, neutral (high energy), greatest penetrating power
Transmutation
When one atomic nuclei is changed into the nucleus of a different element
Natural Transmutation (one reactant)

Artificial Transmutation (two reactants)

Fission (releases energy)
 One atom absorbs a neutron and splits into two or more pieces, giving off a tremendous amount of energy
One atom absorbs a neutron and splits into two or more pieces, giving off a tremendous amount of energy
Fusion (releases more energy)
 When the two light nuclei unite to form a heavier nucleus, fusion creates more energy than fission
When the two light nuclei unite to form a heavier nucleus, fusion creates more energy than fission
Balancing Nuclear Reactions
Set masses of both sides equal
Set Atomic Numbers of both sides equal
Half Life
Time it takes for half of a sample to decay
Divide amount in half for each half life
Uses of Radioisotopes
C-14: used in radioactive dating of once living organisms
U-238: used in radioactive dating of geological (non-living substances)
I-131: used to treat thyroid disorders
Co-60: used in radiation therapy for cancer treatment