Gasses
What is Gas?
Gas Definition in Chemistry: Matter forms into a gas when molecules are moving very fast
No definite shape/volume
Scents are gasses; eventually diffuse
Hot air balloons use gas
Characteristics of Gas
Air is one of the biggest gasses
Mixture composed of many things
Composed of Nitrogen, Oxygen, CO2, NE, HE, CH4, etc.
Noble Gasses
Unreactive
Any liquid/solid can become a gas with enough energy/heat
Gasses fill a container
Can be compressed
Ex: Oxygen in a scuba tank; air in tire
To understand gasses, you need to gain an understand of pressure
Pressure
Definition: The amount of force exerted by molecules against a container
Formula:
Pressure = Force/Area (PSI = Pounds per Square Inch)
Force = Weight/Mass of molecule (lb. or kg)
Area = length * width (in² or cm²)
Not the same pressure as in physics
Area is inversely proportional to pressure
Generally more important than force when determining pressure because force is constant
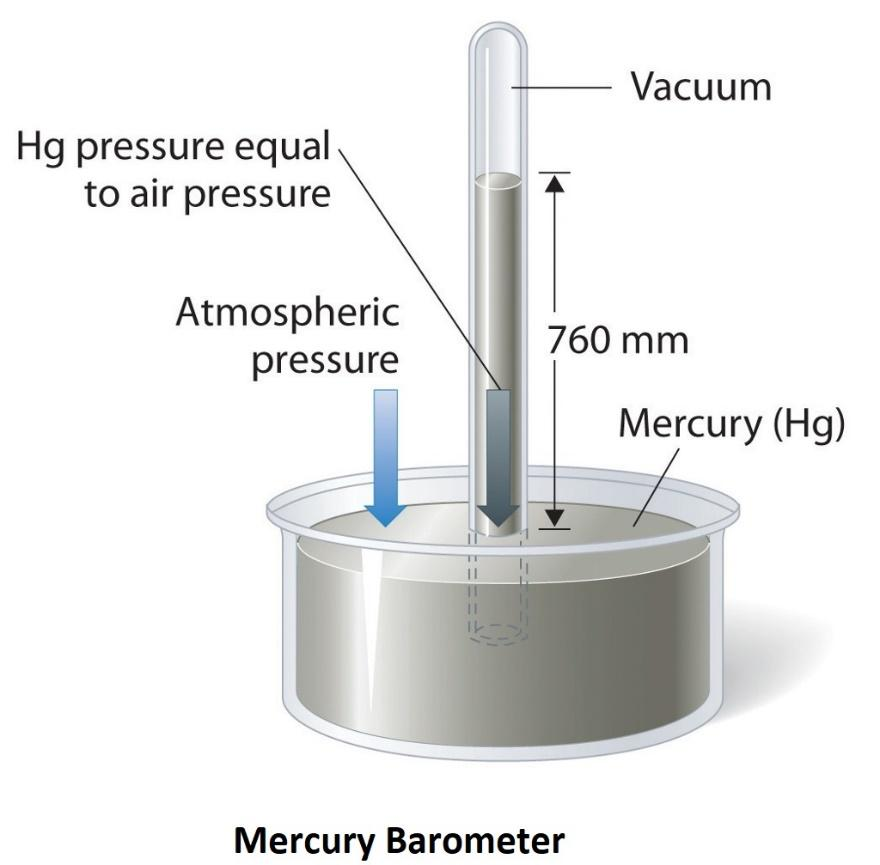
Barometer: Device measuring atmospheric pressure
A mercury barometer measures atmospheric pressure
Mercury rises or falls based on atmospheric pressure by showing how much air pressure pushes down on mercury (Hg), which rises or falls in a tube.
In warm weather, air moves faster and expands, increasing atmospheric pressure.
This increased pressure pushes down more on the mercury in the open dish, causing it to rise in the tube.
The standard atmospheric pressure at sea level is 760 mm Hg
But in warm conditions, the mercury can rise above this as faster-moving air exerts more force on the mercury, leading to a higher column.
Boyles Law
4 Variables needed to determine the state of a gas
Temperature (Constant in Boyles law)
Pressure (Boyles Law)
Volume (Boyles Law)
Number of Molecules (Constant in Boyles law)
Boyles Law: P1V1=P2V2
Robert Boyles Experiment
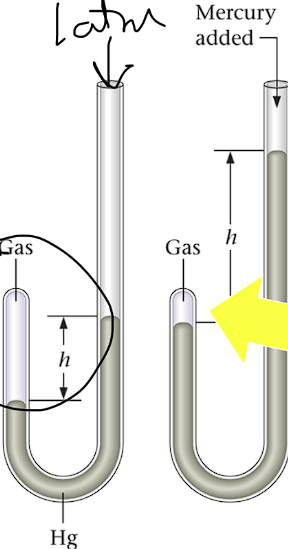
Manometer:
One end sealed
One end open to atmosphere
1 atm is the standard atmospheric pressure on the mercury
Robert Boyle’s Experiment
Tested different volumes effect on pressure using a manometer
Created graph based on his results
High Pressure = Low Volume
High Volume = Low Pressure
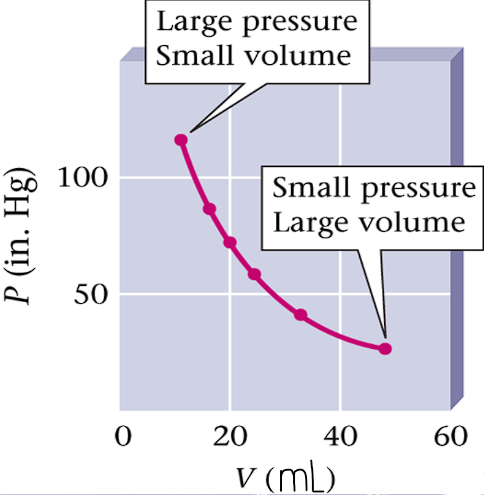
Volume and Pressure are inversely proportional
If one increases, the other decreases
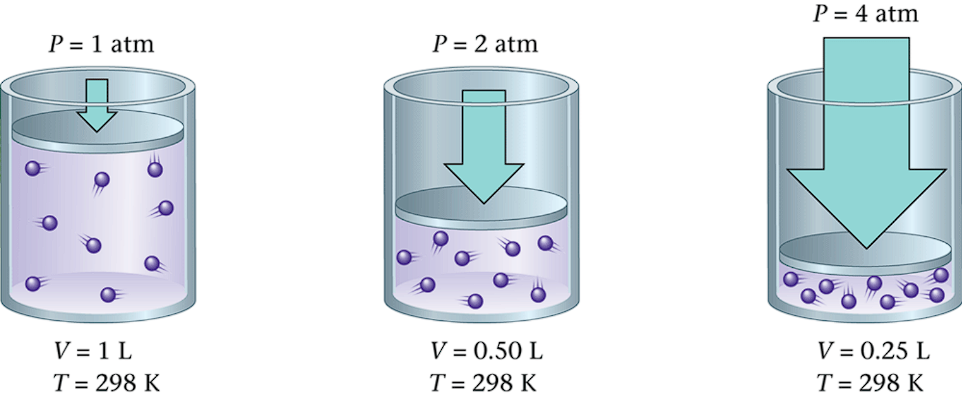
Creates a Constant:
Constant: PV = k
Another way of stating Boyles Law is:
P1V1=P2V2
(For a constant temperature and amount of gas)
Pressure Units must be the same
Practice Problem: A sample of oxygen gas has a volume of 150.0 mL when its pressure is 0.947 atm. What will the volume of the gas be at a pressure of 745 mm Hg if the temperature remains constant?

Boyles Law Demonstration Notes
Use ACE (Answer, Cite, Explain)


Charles Law
Demo Experiment:
We put an Erlenmeyer flask with water on a hot plate and a balloon on top sealing it
What happened?
The water started boiling
Temperature increase
The balloon expanding
Volume increase
What variables are constant?
Amount of gas
Constant because it’s a closed system; nothing enters or leaves)
Pressure
Constant because volume is changing
Direct Relationship: Temperature Increase = Volume Increase
Holding Pressure and number of molecules constant
Volume varies with temperature
They are directly proportional
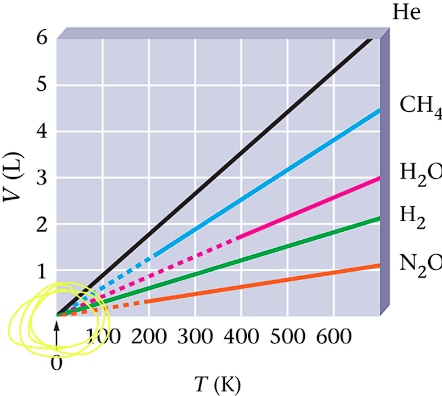
Graphing Data for Several Gasses
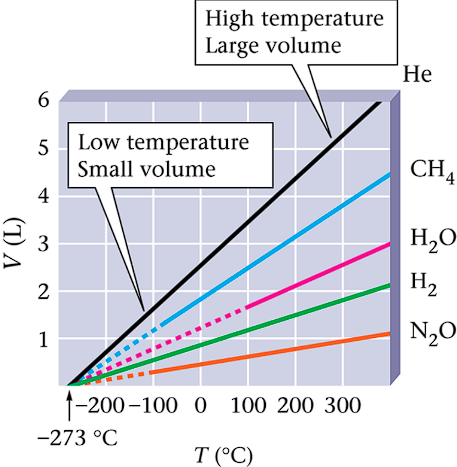
Observe starting point of temperature in graph
-273 degrees Celsius
All gasses start at -273 Celsius
Point of absolute zero temperature
Volume and Temperature: Charles Law
V1/T1 = k
Volume and Temperature are directly proportional
If one increases the other increases
Another way of stating Charles Law is
V1/T1 = V2/T2
(constant pressure and amount of gas)
Charles Law Origin
Discovered by Jacques Charles in 1787
Found volume changes by 1/273 of each original volume for each Celsius degree, at a constant P and initial T of 0 degrees C
This temp. = -273.15 is called absolute zero and given a value of 0 in the Kelvin scale
K = 273.15 + degrees in Celsius
Charles Law applies to Kelvin temperature also
Practice Problems
Problem 1: A sample of neon gas occupies a volume of 752mL at 25 0 C. What volume will the gas occupy at 50 degrees C if the pressure remains constant?


Gay-Lussac Law
P1/T1=P2/T2
Temperature and Pressure are variables
Volume and number of moles/molecules constant
Direct Relationship (if pressure increases, temperature increases)
Similar to Charles Law
P1/T1 = k
Direct Relationship: Division in equation
Inverse Relationship: Multiplication in equation
Check your Understanding: Explain how you can breath air into your lungs.
Use ACE method
Answer: When the pressure in your lungs is higher than atmospheric pressure, you exhale, and when it is lower than atmospheric pressure, you inhale.
Cite: P1*V1=P2*V2
Explain: When you inhale, your diaphragm contracts, increasing the volume of your lungs, decreasing the pressure inside them. Air rushes in to equalize the pressure, filling your lungs with air. When you exhale, the diaphragm expands, decreasing the volume in your lungs, therefore increasing the pressure and pushing the air out. The atmospheric pressure is 1 atm so when the pressure inside your lungs is higher or lower than 1 atm, it causes you to exhale or inhale.
Example Problem:
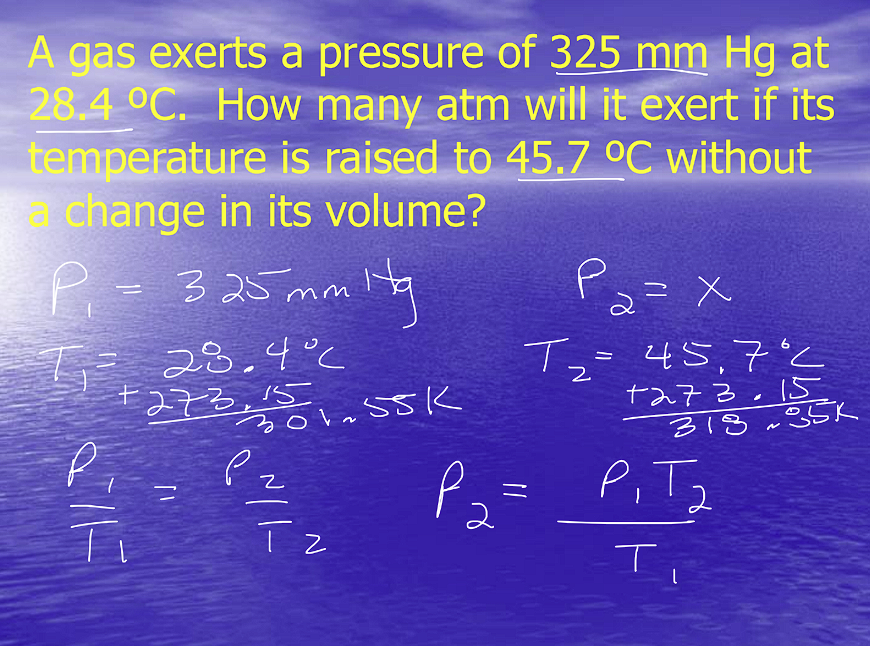
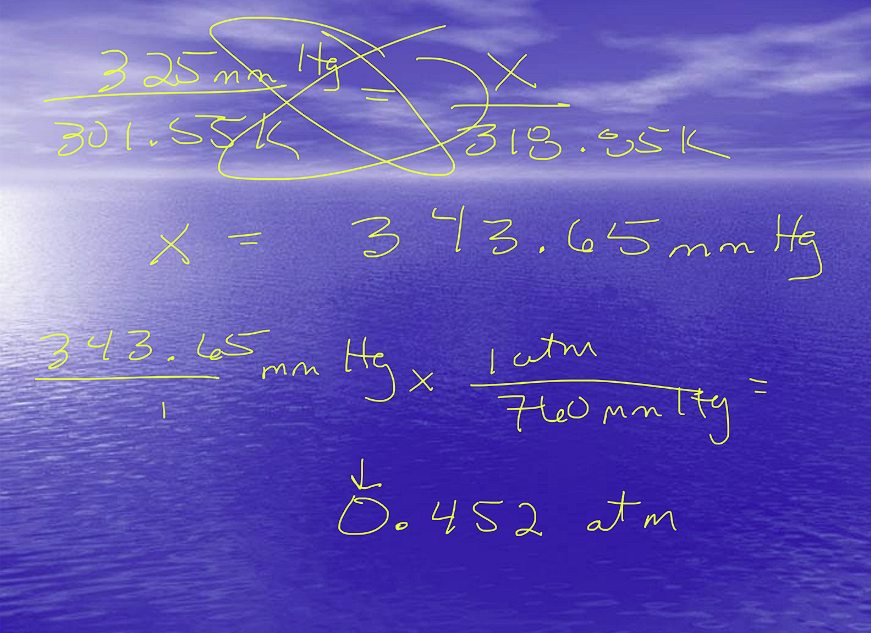
Combined Gas Law
4 Variables needed to know to determine state of a gas
Temperature
Volume
Pressure
Number of moles/molecules
To explain gas interactions, 2 must be constant, while 2 are changing
P1*V1 = P2*V2 (Inverse relationship)
V1/T1 = V2/T2 (Direct relationship)
P1/T1 = P2/T2 (Direct relationship)
Temperature is always on bottom to make the combined gas law equation

Useful for any problem
Temperature musk be in Kelvin (K)
Units must be the same
Practice Problem:

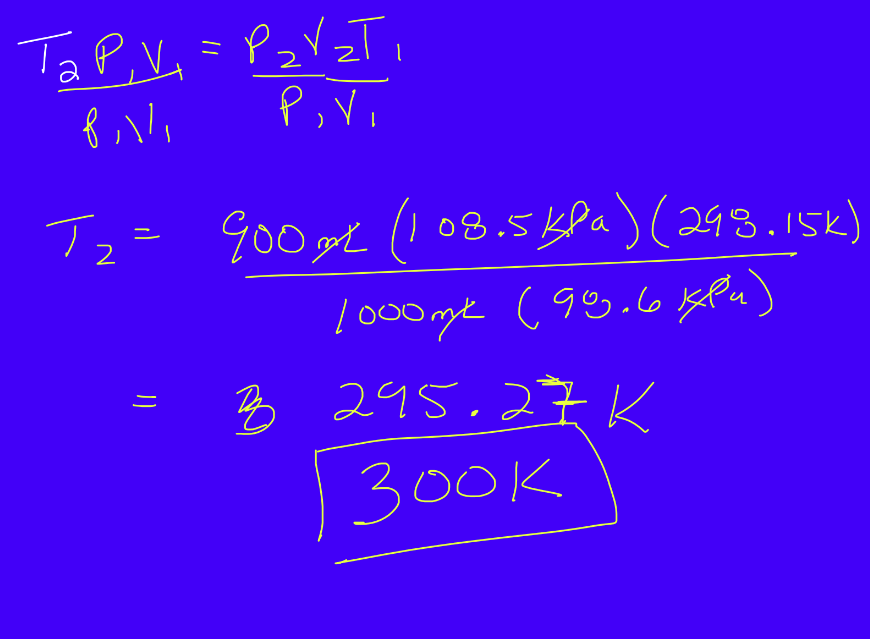
Kinetic Molecular Theory
Gases consist of molecules in continuous random motion.
Volume of all the molecules is negligible compared to total volume of container they are in.
Attractive and repulsive forces between gas molecules are negligible.
Collisions are elastic. Energy can be transferred, but average kinetic energy stays same, as long as temperature is constant.
The average kinetic energy of the molecules is proportional to the absolute temperature.
Application on Gas Laws
What happens when we increase V at constant T and number of molecules?
The increased volume means decreased pressure because the gas particles hit the container less frequently if they are moving at the same speed (temp.
Temperature is the measure of kinetic movement of particles
If two substances have the same temperature, then their average kinetic movement is the same
Units of Pressure
1 standard atmosphere =
1.000 atm
760.0 mm Hg
760.0 torr (named after Torricelli)
101,325 Pa
101.3 kPa
14.7 psi
1000 mL = 1 L
Graphing in Chemistry
Create X, Y, scatter plot
Label the X and Y axis and put the units in
parenthesis, create a title for the graph
Title should be “X vs. Y” or “Boyles Law Graph” in this lab
X axis always longest edge of paper
X is the independent variable – what you
knew when you started the lab
Y is dependent variable – what changed
SCALE – most important part of graph
Scale
Must be easy to figure out
Each line must represent a value so that
you can estimate between lines
For this lab (Boyle’s law lab) each axis, X and Y, start at zero
But not all labs must start at zero
Each scale, X and Y, can be different –
they do not have to be the same