Halogens
Trends in volatility and reactivity of halogens
The halogens are found in Group 7 of the periodic table. Therefore, they have 7 electrons in their valence shell and can accept one electron to form ions with a -1 charge
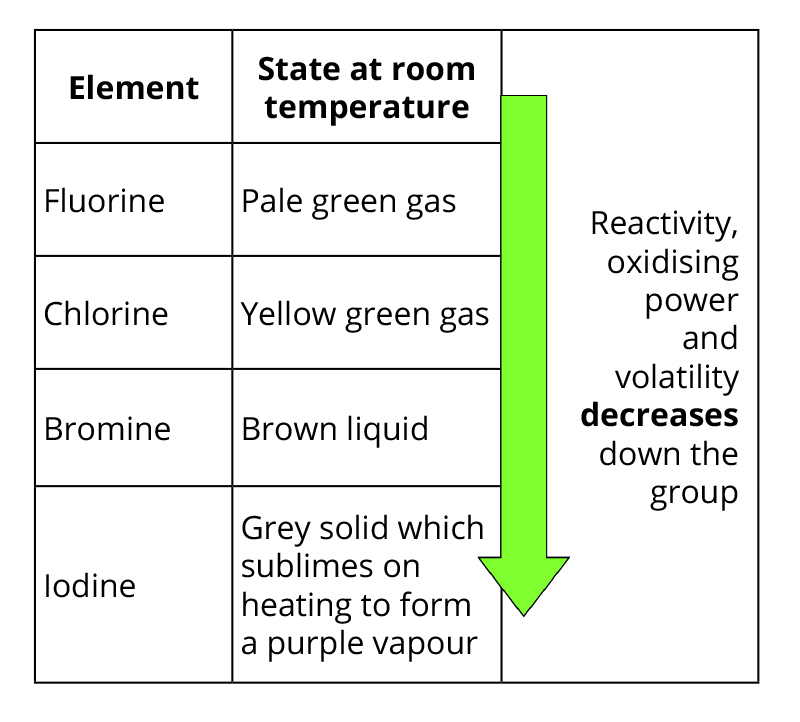
Volatility (tendency to form a gas) and melting point decrease down the group because intermolecular forces strengthen as atoms get bigger.
Larger atoms have more electrons, increasing induced dipole–induced dipole interactions, making the molecules harder to separate.
Reactivity decreases down the group due to a decrease in oxidising power—larger atoms are less effective at attracting electrons. (As they have more electrons already).
Reactions of the halogens with metals
Halogens react with sodium to form sodium halides:
Reaction is highly exothermic and can be explosive.
Produces a bright orange flame (from ignited sodium) and a white solid halide.
General equation:
2Na + X2 → 2NaX
Where X=halide
Halogens react with iron to form iron halides:
Iron wool glows and burns in halogen vapour, producing a brown gas.
Reactivity decreases down the group, requiring heating for lower halogens.
Fluorine, chlorine, and bromine form iron(III) halides
2Fe + 3X2 → 2FeX3
where X=halideIodine is the exception. As it is much less reactive it needs to be heated quite strongly and will only form iron(II) iodide.
Fe + I2 → FeI2
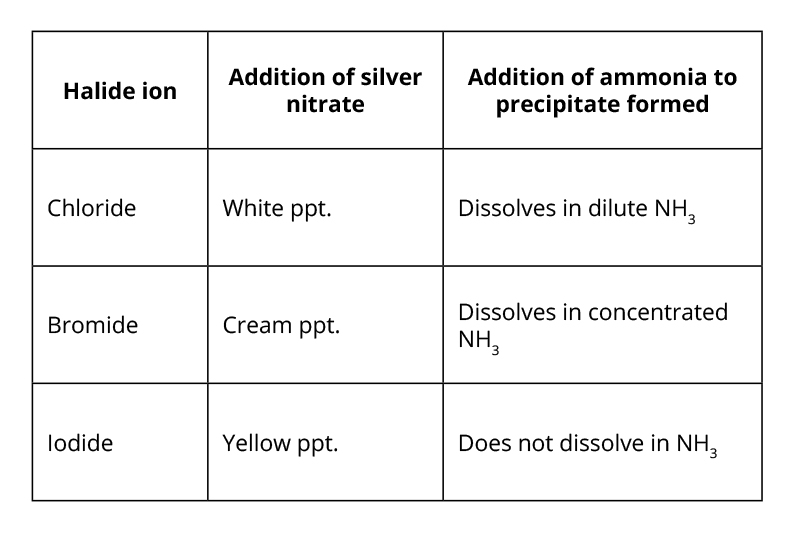
Testing for halide ions.
The reaction of halide ions with silver ions in dilute nitric acid is important in qualitative analysis – identifying unknown solutions or solids.
Test:
First add aqueous sodium hydroxide NaOH (aq).
add dilute nitric acid, HNO3(aq) followed by aqueous silver nitrate, AgNO3(aq)
This will yield a silver halide precipitate as shown by the ionic equation:
X−(aq) + Ag+(aq) → AgX(s)
Where X = the halide.
Further testing with aqueous ammonia, NH3, can be used to distinguish it.
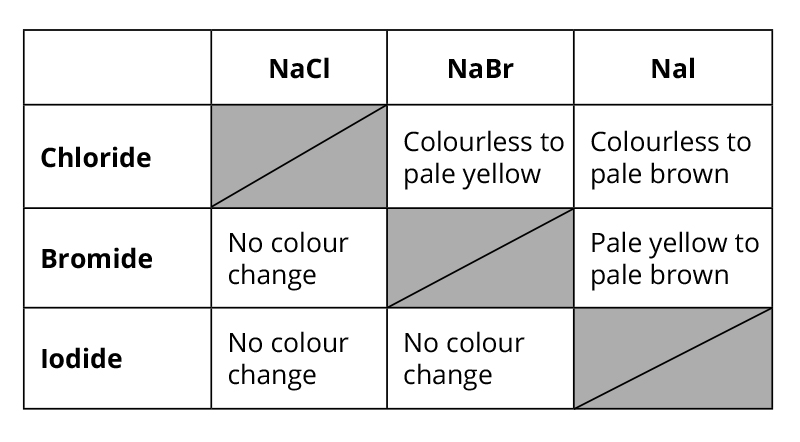
Displacement reactions
The displacement reactions are another set of reactions that we can use to show how reactivity of the halogens decreases down the group.
A more reactive halogen will displace a less reactive one from a solution of one its salts.
Chlorine and fluoride ions in water treatment
Chlorine is added to drinking water to kill bacteria and viruses, preventing diseases like cholera and typhoid.
Forms an equilibrium in water: Cl2+H2O⇌HOCl+HCl
Safe concentration: Below 1 ppm.
Widely accepted due to its health benefits.
Fluoride is added to toothpaste and water to strengthen teeth and prevent decay.
Also helps strengthen bones and may prevent osteoporosis.
Safe concentration: Below 1 ppm.
Higher doses can cause fluorosis (tooth discoloration) and have been linked to bone cancer and thyroid issues.
Controversial: Some see it as forced medication, while others argue toothpaste alone is sufficient.
Oxidising power decreases down the group.