Chapter 3: States of Matter
Chapter Outline
Kinetic Molecular Theory
Forces of Attraction
Intramolecular Forces
Intermolecular Forces
Liquids and Solids
Phase Changes
Chapter 3.1: States of Matter
Kinetic Molecular Theory
The kinetic theory of matter provides an explanation for why matter exists in different phases (solid, liquid, gas) and how it changes from one phase to another. It also helps to understand various properties of matter.
Postulates of the Kinetic Molecular Theory
Matter consists of tiny particles called molecules.
These particles are in constant motion and have kinetic energy.
They possess potential energy due to attractive or repulsive forces among them.
Average particle speed increases as temperature rises.
Energy transfers between particles during collisions without net loss in the system.
Phase Properties of Matter
Property | Solid | Liquid | Gas |
|---|---|---|---|
Particles | Tightly packed atoms or molecules | Closer but less packed atoms or molecules | Widely spaced atoms or molecules |
Energy Movement | Low energy - vibrate around fixed points | Moderate energy - move around | High energy - constantly moving |
Spaces Between | Very little space | Bigger than solids, smaller than gases | Large spaces |
Attractive Forces | Very strong forces - fixed volume | Weaker than solids, stronger than gases | Weak forces - large distance |
Phase Changes | Solids transform to liquids or gases when heated | Liquids transform to gases when heated | Gases turn into liquids or solids when cooled |
Fluid - Liquid
Disperse - Gas
Compact - Solid
Forces of Attraction
Types of Attractive Forces
Intramolecular Forces: Forces that hold atoms together within a molecule (also known as chemical bonds).
Intermolecular Forces: Forces that exist between molecules.
Intramolecular Forces of Attraction
Types of Chemical bonds:
Ionic Bond: Formed by complete transfer of valence electrons between atoms, creating charged cations and anions. (Metal & Non-metal)
Covalent Bond: Formed between atoms with similar electronegativities; electrons are shared. (Non-metal & Non-metal/Metalloid)
Metallic Bond: Occurs between metal atoms where valence electrons are delocalized. (Metal & Metal)
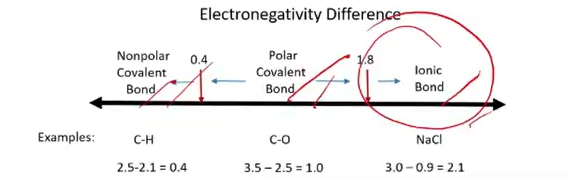
Types of Covalent Bonds
Nonpolar Covalent Bond: Formed between atoms with very similar electronegativities (difference less than 0.4).
Polar Covalent Bond: Formed between atoms with a moderate electronegativity difference (0.5 to 1.8).
— Simpler Version of Types of Bonds —
1. Metallic Compounds:
Composed of metal atoms.
Metals share their outer electrons, creating a "sea of electrons" that move freely.
These compounds are shiny, good conductors of electricity, and malleable.
Example: Copper (Cu), Iron (Fe)
2. Ionic Compounds:
Formed between metals and non-metals.
Metals lose electrons to become positively charged (cations), and non-metals gain electrons to become negatively charged (anions).
The opposite charges attract, creating a strong bond.
Example: Sodium chloride (NaCl), Magnesium oxide (MgO)
3. Polar Covalent Compounds:
Formed between non-metal atoms with different electronegativities.
Electrons are shared, but unequally, creating a partial positive charge on one atom and a partial negative charge on the other.
Example: Water (H₂O), Hydrogen fluoride (HF)
4. Non-Polar Covalent Compounds:
Formed between non-metal atoms with similar electronegativities.
Electrons are shared equally, so there are no charges.
Example: Oxygen (O₂), Methane (CH₄)
Relative Strength of Intramolecular Forces
Type of Bond | Basis of Formation | Relative Strength |
|---|---|---|
Metallic Bond | Metal cations to delocalized electrons | Strongest (1) |
Ionic Bond | Cations to anions | Strong (2) |
Polar Covalent Bond | Partially charged cation to anion | Moderate (3) |
Nonpolar Covalent Bond | Nuclei to shared electrons | Weakest (4) |
Intermolecular Forces of Attraction
These forces are significantly weaker than intramolecular forces but crucial for physical properties like boiling/melting points.
Types of Intermolecular Forces
Dipole-Dipole Interactions: Occur between oppositely charged parts of neighboring polar molecules.
Example: The attraction between hydrogen chloride (HCl) molecules, where the hydrogen (positive) end of one molecule is attracted to the chlorine (negative) end of another molecule.
Only happens in Polar Covalent Bond
Hydrogen Bonding: A strong dipole-dipole interaction occurring between hydrogen and highly electronegative atoms. This is a special type of dipole-dipole interaction that occurs when hydrogen is bonded to a highly electronegative atom like nitrogen, oxygen, or fluorine. This results in a strong attraction between the hydrogen atom of one molecule and the electronegative atom of another.
Example: The attraction between water (H₂O) molecules, where the hydrogen atom of one water molecule is attracted to the oxygen atom of another water molecule. This is why water has such unique properties, like a high boiling point and surface tension.
London Dispersion Forces: These are weak, temporary attractions that occur when electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. These forces are present in all molecules, whether they are polar or non-polar.
Example: The attraction between molecules of nitrogen (N₂) or methane (CH₄) in the liquid phase. Although these molecules are non-polar, the temporary dipoles allow them to attract each other weakly.
All compounds can occur
Momentary collisions
Relative Strength of Intermolecular Forces
Type | Occurs Between | Relative Strength |
|---|---|---|
Hydrogen Bonding | Between H and F, O, N | Strongest of dipole-dipole (1) |
Dipole-Dipole Interaction | Partially charged molecules | Strong (2) |
London Dispersion Forces | Temporary dipoles | Weakest (3) |
Chapter 3.2: Liquids and Solids
Behavior of Liquids
As pressure on gas increases, molecules condense into liquid due to intermolecular attraction, leading to random positioning and irregular space.
Intermolecular Forces in Liquids
Surface Tension: Amount of energy needed to increase the surface area of a liquid. Stronger intermolecular forces increase surface tension, making it harder to break the liquid surface.
Higher ST = Higher IMFA
Viscosity: Measure of a fluid's resistance to flow; higher intermolecular attraction means higher viscosity.
Properties of Solids
Cooling of molecules leads to a solid formation when attractive forces become dominant, creating a crystalline structure (rigid and ordered arrangement) or amorphous structures (lacking defined order).
Solids can be divided into two categories:
Crystalline Solids - The forces responsible for the stability of any crystal can be ionic forces, covalent bonds, van der Waals forces, hydrogen bonds, or a combination of these forces. Distinct melting points, sharp edges, flat surfaces due to their regular arrangement.
Amorphous solids, such as glass, lack a well-defined arrangement and long-range molecular order. These do not have distinct melting points; soften over a range of temperatures.
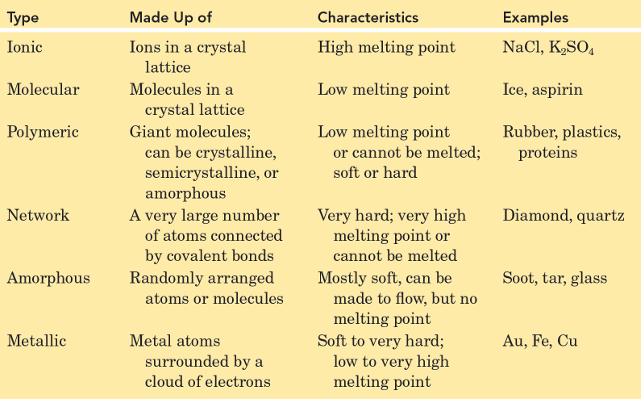
Ionic - Made up of positive and negative ions. Held by together by strong ionic bonds. High melting and boiling point, hard and brittle, conduct electricity when melted or dissolved in water.
Molecular - Made up of moleculres held together by intermolecular forces (Dipole-diople, hydrogen bonding, london dispersion) These are forces weaker than ionic and covalent bonds. Low melting and boiling points, usually soft, poor conductors of electricity.
Polymeric - Made up of long chains of repeating units. Covalents bond within the chains and the London Dispersion Forces or Hydrogen bonds between the chains. Can be flexible or rigid, depending on the type of polymer; generally low melting points to ionic and metallic solids.
Network- Made up of atoms connected by covalent bonds in a continous network. Strong covalent bonds throughout the netweok. High melting and boiling points, very hard, usually poor condutors of electricity.
Metallic - Made up of metal atoms. The metallic bonds where electrons are free to move throughout the structue. (Sea of electrons). Good conductors of electricity and heat, malleable, ductile, usually have high melting and boiling points.
Physical Properties of Matter
Compressibility: Change in volume due to pressure change.
Thermal Expansion: Change in volume due to temperature change.
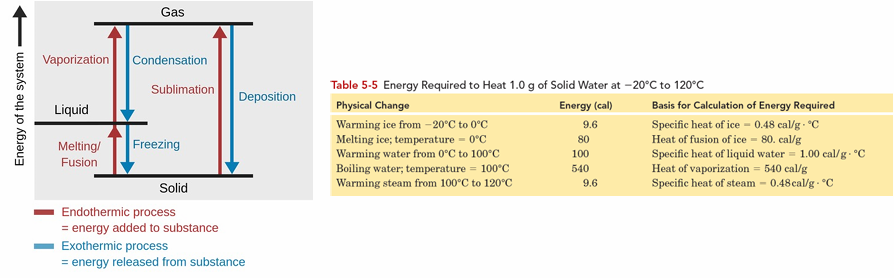
Phase Changes
Energy is required for phase transitions to overcome intermolecular forces.
Refer to the transformation of a substance from one state of matter to another.
Common phase changes include: Melting, Freezing, Condensation, Vaporization, Sublimation, and Deposition.
Triple Point: A specific point in a phase diagram where all three states of matter coexist. For water, this occurs at 0.01 degrees celcius and 611.657 pascals of pressure.
Phase Diagram: Graphical representation of the physical states of a substance under varying temperature and pressure. It helps understand at what conditions these changes occur.
Solid: Lower Left
Liquid: Middle
Gas: Lower right
Critical point: The temperature and pressure at which a substance can no longer exist as a liquid and gas phases become indistinguishable.
Boiling Point/Melting Point guide
Element < Diatomic Molecule < NonPolar/London Dispersion < Dipole-dipole < Ionic < Hydrogen Bond < Metallic
If same group, depend it on the electronegativity difference.
If all are ionic compounds, base it on the number of nonmetals; the higher it is, the higher the boiling/melting point.
 Knowt
Knowt