Chapter 13 - Molecular Spectroscopy 1: Rotational and Vibrational Spectra
General features of spectroscopy
- Emission spectroscopy - A molecule undergoes a transition from a state of high energy E1 to a state of lower energy E2 and emits the excess energy as a photon.
- Absorption spectroscopy - The net absorption of nearly monochromatic (single frequency) incident radiation is monitored as the radiation is swept over a range of frequencies.
- Stokes radiation - When incident photons collide with the molecules, give up some of their energy, and emerge with a lower energy.
- Anti-Stokes radiation - When incident photons collect energy from the molecules (if they are already excited), and emerge with a higher frequency.
13.1 Experimental techniques
- Spectrometer - An instrument that detects the characteristics of light scattered, emitted or absorbed by atoms and molecules.
- Dispersing element - It separates radiation into different frequencies.
13.2 The intensities of spectral lines
Transmittance - The ratio of the transmitted intensity to the incident intensity at a given frequency.
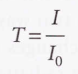
Beer-Lambert law
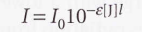
Absorbance

Beer-Lambert law with absorbance
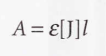
Integrated absorption coefficient - The sum of the absorption coefficients over the entire band.
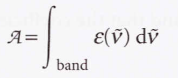
Absorption intensities
Stimulated absorption - The transition from a low energy state to one of higher energy that is driven by the electromagnetic field oscillating at the transition frequency.
Transition rate
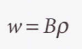
Total rate of absorption (W) - The transition rate of a single molecule multiplied by the number of molecules N in the lower state.
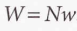
Selection rules and transition moments
- Gross selection rule - It specifies the general features a molecule must have if it is to have a spectrum of a given kind.
- Specific selection rules - They express the allowed transitions in terms of the changes in quantum numbers.
13.3 Linewidths
- Doppler effect - In which radiation is shifted in frequency when the source is moving towards or away from the observer.
- Collisional deactivation - Arises from collisions between molecules or with the walls of the container.
- Collisional lifetime - The mean time between collisions.
Pure rotation spectra
13.4 Moments of inertia
Moment of inertia - The mass of each atom multiplied by the square of its distance from the rotational axis through the center of mass of the molecule.

Rigid rotors - Bodies that do not distort under the stress of rotation.
- Spherical rotors - They have three equal moments of inertia.
- Symmetric rotors - Two equal moments of inertia.
- Linear rotors - One moment of inertia.
- Asymmetric rotors - Three different moments of inertia.
13.5 The rotational energy levels
Degeneracies and the Stark effect
- Stark effect - The splitting of states by an electric field.
13.6 Rotational transitions
- For a molecule to give a pure rotational spectrum, it must be polar.
13.7 Rotational Raman spectra
- Gross selection rule for rotational Raman transitions - The molecule must be anisotropically polarizable.
13.8 Nuclear statistics and rotational states
- Nuclear statistics - The selective occupation of rotational states that stems from the Pauli principle.
- Ortho-hydrogen - The form with parallel nuclear spins.
- Para-hydrogen - The form with paired nuclear spins.
The vibrations of diatomic molecules
13.9 Molecular vibrations
Vibrational terms - The energies of its vibrational states expressed in wavenumbers.
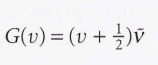
13.10 Selection rules
- Infrared active vibrations - Where the electric dipole moment of the molecule must change when the atoms are displaced relative to one another.
13.11 Anharmonicity
- Anharmonic motion - When the restoring force is no longer proportional to the displacement.
The convergence of energy levels
Morse potential energy
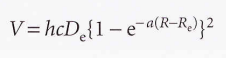
The Birge-Sponer plot
- Birge-Sponer plot - Graphical technique used to determine the dissociation energy of the bond when several vibrational transitions are detectable.
13.12 Vibration-rotation spectra
Spectral branches
- Branches - Groups in which absorptions are divided.
- P branch - All transitions with J = -1.
- Q branch - All lines with J = 0.
- R branch - Lines with J = +1.
Combination differences
- Combination differences - A procedure used widely in spectroscopy to extract information about a particular state. It involves setting up expressions for the difference in the wavenumbers of transitions to a common state.
13.13 Vibrational Raman spectra of diatomic molecules
- Gross selection rule for vibrational Raman transitions - The polarizability should change as the molecule vibrates.
The vibrations of polyatomic molecules
13.14 Normal modes
- Normal mode - An independent, synchronous motion of atoms or groups of atoms that may be excited without leading to the excitation of any other normal mode and without involving translation or rotation of the molecule as a whole.
13.15 Infrared absorption spectra of polyatomic molecules
- Gross selection rule for infrared activity - The motion corresponding to a normal mode should be accompanied by a change of dipole moment.
- Parallel bands - The transitions arising when the dipole moment change is parallel to the principal axis.
- Perpendicular bands - Transitions involving the perpendicular change of a dipole to the principal axis.
- Force field - The set of force constants corresponding to all the displacements of the atoms.
- Tumbling - The random change of orientation in molecules.
13.16 Vibrational Raman spectra of polyatomic molecules
Exclusion rule - If the molecule has a center of symmetry, then no modes can be both infrared and Raman active.
Depolarization ratio - The ratio of the intensities of the scattered light with polarizations perpendicular and parallel to the plane of polarization of the incident radiation.
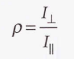
Depolarized line - If the depolarization ratio is close to or greater than 0.75.
Polarized line - If the depolarization ratio is less than 0.75.
Resonance Raman spectroscopy - A modification of the basic Raman effect involves using incident radiation that nearly coincides with the frequency of an electronic transition of the sample.
13.17 Symmetry aspects of molecular vibrations
- If the symmetry species of a normal mode is the same as any of the symmetry species of x, y, or z, then the mode is infrared active.
- If the symmetry species of a normal mode is the same as the symmetry species of a quadratic form, then the mode is Raman active.